SOP FOR ANNUAL PRODUCT QUALITY REVIEW
PURPOSE: To lay down the procedure for preparation, review and approval of annual product quality review (APQR).
SCOPE: This SOP is applicable is applicable to APQRs for products manufactured.
RESPONSIBILITY:
Preparation of SOPs: Officer QA Department
Checking and Review of the SOPs: Officer QA Department
Approval of the SOPs: Executive QA department
Authorization of SOP: Head QA/ His or her Designee.
ACCOUNTABILITY: Head-QA or Designee shall be accountable for the proper implementation & compliance of the SOP.
PROCEDURE:
Definitions:
Annual Product Quality Review: An evaluation, conducted at last annually, to assess the quality standard of each drug product with the objective of verifying the consisting process and appropriateness of current speeds, and highlight any trend, in order to determine the need for changes in drug product specification or of manufacture or general procedure.
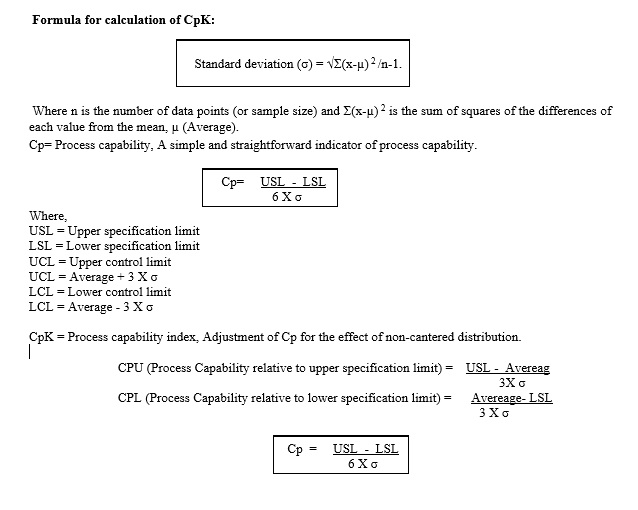
Process capability (Cp): Process capability is a technique to find out the measurable property of a process to a specification. Generally, that final solution of the process capability is specified either in the form of calculation or histograms. Cp is used to evaluate the variation of the process.
Process capability index (Cpk): Process capability index is the measure of process capability It is shows how closely a process is able to produce the output to its overall specifications. Cpk is used to evaluate the centering of the process.
After release of the product, the trend shall be prepared in excel sheet and reviewed for out of trend, If any finally the trend shall be compiled on annual basis.
APQR shall be carried out for each commercialized & exhibit batch of product that area manufactured in the calendar year i.e. January to December. Summary report shall be available within 90 days after the end of calendar year.
APQR shall be prepared by quality assurance department as per SOP.
APQR shall be reviewed by, Quality assurance, production, and quality control departments.
APQR shall be done for product of which minimum 3 Batch executed, and previous year Annual Product Quality Review report shall be clubbed for the product of below 3 batches executed.
If there are less than three (03) batches of product manufactured in calendar year or no previous APQR of respective product are prepared, then these batches shall be covered in next year APQR.
APQR shall be grouped/ clubbed for similar product (i.e. formulation and primary packing is same, however brand name or market is different).
The Annual Product Quality Review of the products related to Loan License or third party can be made as per recommendations of the customers (if required).
Numbering system for APQR: APQR shall be logged in logbook during signing of APQR as per Format No. SOP.
Annual product review shall essentially include the Following information but not limited:
Product Details: Review and record product details like – Product name, Generic name, Label claim, Product description, Pack description, Standard batch size, Shelf life, Review Period (it is based on last period of APQR / Schedule or based on client requirement or annual period), year of review and Number of batches manufactured in the year/period.
Review of Batch Manufactured: Review and record the Batch details like Batch number, Batch size, Manufacturing Date and Expiry Date of all batches manufactured, packed, distributed during the review period. Also mention the details for numbers of batches released, numbers of batches rejected & numbers of validation batches during review period.
Formulation Details: Review and record formulation details like – Material code, Ingredients, use of material and Quantity.
Review of Starting Material (Raw Material): This section shall include Review of raw material used for manufacturing, A.R. No., assay, water content, raw material quantity used, vendor details.
Review of Packaging Material: This section shall include Review of the packaging material used for the packaging and it shall be complied the respective specification.
Review of in Process Test Result: This section shall include Review of process parameter, in process test results of pH, BET, particulate matter and non-viable particles (such as aseptic assembly, connections of equipments, filling, loading, unloading and sealing.)
Review of Finished Product Test Result: This section shall include review as finished product test result parameters such as, Assay, pH, Water Content / LOD, Sub Visible Particulate Matter, sterility, impurity, final yield and trending for the same shall be done. In case of impurity if any result obtained ND (None detected) & BDL (Below disregard limit) graphical trending shall not be done.
Review of Microbiological Environmental Monitoring: Review of results and methods used for microbiological environmental monitoring of dispensing, manufacturing, filling and packing area.
Review of Control Sample: This section shall include review of control sample details, which has been conducted annually for control samples of each packaging configurations.
Review of Stability Data: This section shall include review of stability results at various conditions / various pack configurations.
Review of Media Fill: Review the media fill report which has been conducted in previous year.
Review of Deviation, CAPA and Process Non-Confirmative: This section shall include Review of all batch related deviation & process related deviation, CAPA and Non-Confirmative that particular period Whenever, CAPAs are recommended, appropriate CAPA Shall be initiated and shall be completed in a timely and effective manner. Recommended CAPA shall be escalated to senior Management through routine Quality review meeting.
Review of Out of Specification: This section shall include review OOS related to the product that have been initiated, reviewed and approved during the year.
Review of Out of trend: This section shall include review OOT related to the product that have been initiated, reviewed and approved during the year.
Review of Market Complaint: This section shall include review of complaints received from the market for the particular product and the field alerts.
Review of Batch Rejections: This section shall include review of number of batches rejected for this product during the review period.
Review of Change Control: This section shall include review of changes carried out related to particular product like, process, analytical methods, specifications, validation and qualification of instruments/ equipments etc.
Review of Recalls: This section shall include review of recall history if any of particular product.
Review of Returned Goods: This section shall include Review of number of batches returned reason of return and other relevant detail.
Validation / Qualification Review: This section shall include review of validation / Qualification status of critical relevant equipments and utilities such as HVAC, Nitrogen etc.
Review of Technical Agreement: Review the Technical Agreement for any outsourced GMP activity with clients, outsourcing testing laboratories and API manufacturer & ensures that it is in compliance with current regulatory requirement.
Review of Recommendation and / or Unresolved Issues from Previous APQR: This section shall include the review of open issues from previous APQR corrective action and preventive actions recommended during previous APQR. shall be verified and ensure the adequacy and compliance. Detail observation shall be included.
Conclusion: Review and compare the observations & results of product data mentioned in protocol with their acceptance criteria. Derive the conclusion and recommendations based on the results & trends of the product data. If any results found out of trend, necessary investigation shall be done to find out the probable cause and corrective preventive action shall be decided for future batches.
Recommendation: Write recommendation based on the results and conclusions. Based on above recommendations as well as changes shall be implemented through change control.
Note: Each parameter of APQR shall be reviewed individually with its review comments.
Statistical data:
Statistical analysis technique “process capability” and ‘process performance” shall be used to review the data collected as part of the APQR.
Process capability and process performance study are statistical method which shall be used to establish if specification limit are set appropriately by calculating of Cp (process capability), Cpk (process capability index).
For non-numerical data, no statistical analysis is necessary like: appearance, solubility etc.
Minimum ten lot or stability data shall consider for process capability study. If data are less than ten in the year than data shall be consider in next annual product quality review.
CpK value shall be calculated for critical process parameter and finish product results.
The purpose of a process capability study is to compare specification to the process output and determine statistically if the process can meet the specification.
In case Lower Control limit and upper control limit exceed the Lower specification limit and upper specification limit than Lower specification limit and upper specification limit consider as 3s limit.
In case lower specification limit is not available then the lower specification limit shall consider as zero (0).
In case upper specification limit is not available therefore Cpk value shall found in negative value and the negative Cpk value shall not consider for process capability so that the CPL value considered as Cpk value.
CpK Value:
| CpK Value | Action taken |
| Below 1.0 | Shall be recommended for improvement. |
| Between 1.0 to 1.33 | Shall be recommended for further trending during APQR of next year. |
| Higher than 1.33 | Has been meant that the process parameter is rugged and manufacturing process is capable for producing product. |
Note: If any parameter found out of above acceptance criteria the same shall be reviewed and investigated. The QA shall suggest the further course of action based on the outcome of the investigation.
CpK value shall be calculated for critical process parameters and critical quality attributes.
Note: Other department head shall be responsible to provide the required data to Quality Assurance department for preparation of APQR.
REFERENCE:
SOP FOR SOP
WHO-TRS 961 Annexure-3
Guideline Notes on Product Quality review January 2013 Guide-MQA-024-004.