UV LAMP EFFICACY TEST WHAT IS UV LIGHT? The light with a wavelength of 10-400 nano meters (nm) in sunlight is called ultraviolet light, and it can be divided into UVA, UVB, and UVC (200 nm to 275 nm) according to different wavelength bands. Among them C-band ultraviolet rays destroy …
Read More »UV efficacy Challenge test
UV efficacy Challenge test Evaluation of ultraviolet irradiation efficacy in an automated system for the aseptic compounding using challenge test Objective Ultraviolet (UV) irradiation efficacy in the intravenous compounding robot APOTECA chemo was evaluated to define the best operative conditions in terms of sterility and time optimization. Design The challenge …
Read More »Sterilization (Introduction, Methods, Definition of Terms)
Sterilization (Introduction, Methods, Definition of Terms) Introduction A major risk of all such medical and surgical instruments procedures is the introduction of pathogens that can lead to infection. Failure to properly disinfect or sterilize equipment carries not only risk associated with breach of host barriers but also risk for person-to-person …
Read More »List of Essential Medicines
List of Essential Medicines (W): Included in WHO Model List of Essential Medicines (I): Included in National List of Essential Medicines (2011), India A Abacavir (ABC) (W) Acenocoumarol (I) Acetazolamide (W,I) Acetic acid (W) Acetylcysteine (W) Acetyl salicylic acid (Aspirin) (W,I) Acriflavin + Glycerine (I) Actinomycin D (Dactinomycin) (W,I) Activated …
Read More »PROTEINS & TYPES OF PROTEINS
PROTEINS Proteins are high molecular weight polyhydroxy peptides containing alpha amino acids joined together by peptide linkage (bond) C―CO―Na. They contain ―C, H, N and S sometimes phosphor also. The molecular weight may range from 6000 to many millions. Proteins are made up of amino acids which from the fundamental …
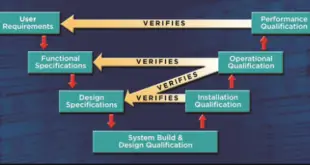
Read More »Qualification & Validation of Equipment, Systems, Utilities in Pharmaceuticals
Qualification & Validation of Equipment Equipment qualification as required by the FDA requires verification documentation to start with the Validation Master Plan (VMP) and flow through a series of documents that define the scope and tasks required to successfully execute your equipment qualification task. The VMP dictates the actions that all persons involved …
Read More »PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM
PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM OBJECTIVE To describe the Performance Qualification procedure to be used during validation of purified water system in order to: a) ensure the system reproducibility over an appropriate time period as per user requirement specifications. b) ensure that the system is showing consistency in producing …
Read More »Cleaning Validation – Glossary of Terms
Cleaning Validation Glossary of Terms 1 Acceptable daily intake An amount of a substance administered or consumed on a daily basis that will not produce a pharmacological or toxic response 2 Analyte Substance for which an analysis is being performed 3 API Active pharmaceutical ingredient 4 Automated cleaning A cleaning …
Read More »Production & purification of drinking-water as per WHO
Production & purification of drinking-water as per WHO General considerations The specifications for WPU found in compendia (e.g. pharmacopoeias) do not define the permissible water purification methods apart from for BWFI. The chosen water purification method or sequence of purification steps must be appropriate to the application in question. The …
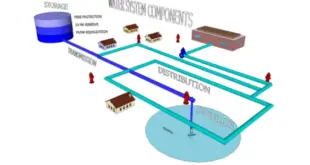
Read More »General principles for pharmaceutical water systems As per WHO
General principles for pharmaceutical water systems Pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualified and maintained to ensure the reliable production of water of an appropriate quality. It is necessary to validate the water production process to ensure the water generated, stored and distributed is …
Read More »Materials in Pharmaceutical as per WHO Guideline
Materials in Pharmaceutical as per WHO Guideline Principle. The main objective of a pharmaceutical plant is to produce finished products for patients’ use from a combination of materials (starting and packaging). Materials include starting materials, packaging materials, gases, solvents, process aids, reagents and labelling materials. No materials used for operations …
Read More »SOP on SOP
SOP on SOP SOP on SOP is a Guidance of SOP that will explain how to draft and prepared the SOP, A standard operating procedure (SOP) is a step-by-step, repeatable process for any routine task. It’s a kind of documentation that prevents stress, mistakes, and miscommunication. SOPs ensure reliability, efficiency, …
Read More »What are good documentation practices & how can they best be implemented?
What are good documentation practices & how can they best be implemented? Good documentation practice (commonly abbreviated GDP, recommended to abbreviate as GDocP to distinguish from “good distribution practice” also abbreviated GDP) is a term in the pharmaceutical and medical device industries to describe standards by which documents are created and maintained. While some GDP / GDocP standards …
Read More »Drug Master Files (DMFs) and it submission
Drug Master Files (DMFs) and it submission Drug master files (DMFs) are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. They: Allow parties to reference material without disclosing DMF contents to those …
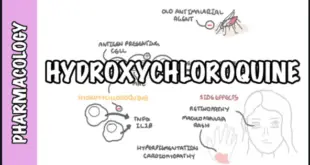
Read More »Hydroxychloroquine and Antimalarial Drugs (united healthcare)
Hydroxychloroquine and Antimalarial Drugs (united healthcare) These are antimalarial drugs found to induce remission in upto 50% patients of Rheumatoid Arthritis (RA), but take 3–6 months. Their advantage is relatively low toxicity, but efficacy is also low; bony erosions are not prevented. Their mechanism of action is not known, however, …
Read More »Characterization of Fine Particle in Formulation of drugs
Characterization of Fine Particle in Formulation of drugs Parameters those are measured: (i) Particle size and size-distribution (ii) Shape of the particle (iii) Surface morphology of the particles (iii) Zeta potential Instrumental Methods of Particle Size Characterization: (i) Light Microscope: • First a standard graticule (BS 3625) is standardized with …
Read More »PROCESSING OF PARENTERAL PREPARATION
PROCESSING OF PARENTERAL PREPARATION The following steps are involved in the processing of parenteral preparation: 1. Cleaning of containers, closures, and equipment. 2. Collection of materials. 3. Preparation of parenteral products. 4. Filtration. 5. Fill the preparation in the final container. 6. Sealing the container. 7. Sterilization 8. Evaluation of …
Read More »Formulation of Suspensions
Formulation of Suspensions Biphasic liquids such as suspensions and emulsions are unique dosage forms because many of their properties are due to the presence of a boundary region between two phases. In suspensions, a liquid and an insoluble solid meet to form an interface. In the case of emulsions, two …
Read More »Determination of equilibrium solubility of a drug
Determination of equilibrium solubility of a drug Determination of equilibrium solubility of a drug: The drug is dispersed in a solvent. The suspension is agitated at a steady temperature. Samples of the suspension are withdrawn as a function of time, clarified by centrifugation,and assayed to establish a plateau concentration. Solvents …
Read More »QC SOPs List
QC SOPs List SNo. SOPs Title 1.0 SOP on entry & exit procedure in Quality Control Department 2.0 SOP on sampling of raw material. 3.0 SOP on intermediate and finished product analysis and approval 4.0 SOP on analysis of sample by contract laboratory 5.0 SOP on sampling procedure of packaging …
Read More »