CLINICAL TRAILS
- To collect data on usual and unusual events, conditions, & population groups.
- To test hypotheses formulated from observations.
- To understand better one’s
Three main types are there
Observational Studies:
- Groups are studied & contrasts made between groups
- The observed data collected are analyzed
Analytic Studies:
Also called Experimental
Study the impact of a certain therapy
Ultimately the investigator controls factor being studied
Clinical Trial:
Considered the “true” experimental study
“Gold Standard” of clinical research
Often a prospective study that compares the effect and value of an intervention against a control in human subjects
The Different Study Designs
- Case-control
- Cohort
- Case Reports
- Case Series
- Outcomes Based:
- Survey Research:
Quality of Life Questionnaires
Decision analysis Polls
Economic Analysis Surveys
- Meta Analyses
- Survival Analysis
- Randomized Clinical Trial
Terminology
- Cross-sectional Study: Presence or absence of exposure to possible risk factor measured at one point in time. Prevalence obtained.
- Prevalence: The # of new cases and existing cases during specified time period.
- Incidence: The # of NEW cases per unit of a population at risk for disease occurring during stated time period.
Historical Highlights of Drug Trials
- 1909: Paul Ehrlich – Arsphenamine
- 1929: Alexander Fleming – Penicillin
- 1935: Gerhard Domagk – Sulfonamide
- 1944: Schatz/Bugie/Waksman – Streptomycin
- By 1950, the British Medical Res. Council developed a systematic methodology for studying & evaluating therapeutic interventions
Components of Clinical Trials
- Involve human subjects
- Move forward in time
- Most have a comparison CONTROL group
- Must have method to measure intervention
- Focus on unknowns: effect of medication
- Must be done before medication is part of standard of care
- Conducted early in the development of therapies
Clinical Trial Designs
- Randomized/blinded trial
- Randomized/double blinded trial
- Non-randomized concurrent controlled trial
- Placebo trial
- Historical controlled trial
- Crossover Trial
- Withdrawal trial
Simplified
- Randomized: Schemes used to assign participant to one group
Ex: Every 3 gets higher dose
- Nonrandomized: All with Hep. C = cases; others = controls
- Protocol: Study design – instructions
Blinded: Participants do not know if in experimental or control group
Double Blinded: Participants AND staff do not know group assignment
Placebo: Inactive pill with no therapeutic value
Components of Clinical Trial Protocols
- Investigating two or more conditions so have two(+) groups
- Ex: drug vs. placebo; medicine vs. surgery; low dose vs. high dose
- Specific inclusion/exclusion criteria
- Sample size & power calculations
- Plan re: potential biases
- Plan re: handling of attrition/loss to follow up
Study Participant Recruitment
- Identify eligible participants
- Explain study
- Provide informed consent
- Reassess eligibility
- Assign to one group
Participants should be told:
- May have side effects (adverse effects)
- Time commitment
- Benefits & risks
- May withdraw at any time
- Enrollment 100% voluntary
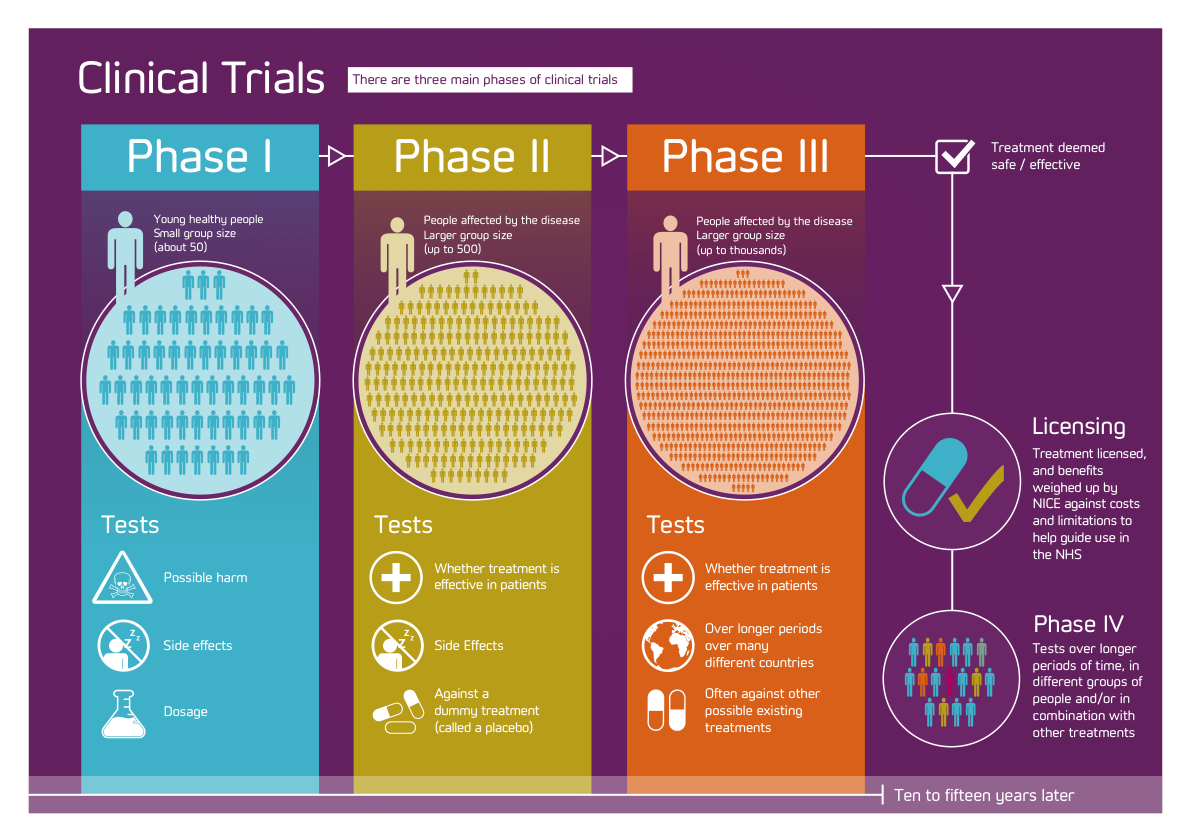
Phases of Clinical Trials
- Most trials that involve new drugs go through a series of steps:
–#1: Experiments in the laboratory
–#2: Once deemed safe, go through 1-4 phases
- Phase I: Small group [20-80] for 1st time to evaluate safety, determine safe dosage range & identify SE
- Phase II: Rx/tx given to larger group [100-300] to confirm effectiveness, monitor SE, & further evaluate safety
- Phase III: Rx/tx given to even larger group [1,000-3,000] to fulfill all of Phase II objectives & compare it to other commonly used txs & collect data that will allow it to be used safely
- Phase IV: Done after rx/tx has been marketed – studies continue to test rx/tx to collect data about effects in various populations & SE from long term use.
Clinical trails at a glance
| # Subs. | Length | Purpose | % Drugs Successfully Tested | |
| Phase I | 20 – 100 | Several months | Mainly Safety | 70% |
| Phase II | Up to several 100 | Several months- 2 yrs. | Short term safety; mainly effectiveness | 33% |
| Phase III | 100s – several 1000 | 1-4 yrs. | Safety, dosage & effectiveness | 25-30% |
Ethics of Clinical Trials:
Protection of Participants
3 ethical principles guide clinical research:
- Respect for Persons: Treatment of person as autonomous
- Beneficence: Issue re: potential conflict between good of society vs. individual
- Justice: Treatment of all fairly & all equally share benefits & risks
Ethical Norms of Clinical Trials
Sound study designs take into account:
- Randomization or sharing of risks
- Proper use of placebo
- Processes to monitor safety of rx/tx
- Competent investigators
- Informed consent
- Equitable selection of participants
- Compensation for study related injuries
Ethical Issues: Protection of Human Subjects
- Rely on integrity of Investigator but outside groups also have oversight
- Participants’ rights protected by Institutional Review Boards [IRBs]
An IRB is defined as: “any board, committee or other group formally designated by an institution to review, to approve the initiation of, and to conduct periodic review of biomedical research involving human subjects”
Human Subjects’ Protection
IRB responsible for such tasks:
- Review research to ensure that potential benefits outweigh risks
- Develop and issue written procedures
- Review research for risk/benefit analysis & proper protection of subjects
- Issue written notice of approval/disapproval to the Investigator
- Review and respond to proposed protocol changes submitted by the Investigator
- Review reports of deaths, and serious and unexpected adverse events received from the Investigator.
- Conduct periodic continuing review of the study, study risks, selection of subjects, privacy of subjects, confidentiality of data, and the consent process.
10 Key Points to be Noted
- Voluntary informed consent
- Experiment must be for the good of society, & results not obtainable by other means
- Experiment should be based upon prior animal studies
- Physical & mental suffering & injury should be avoided
- No expectation that death/disabling injury will occur from the experiment
- Risk vs. benefit
- Protect subjects against injury, disability, or death
- Only scientifically qualified persons to be involved
- Subject can terminate her/his involvement
Informed Consent:
A Part of Human Subject Protection
Objectives of Informed Consent
To Ensure:
- Voluntariness
- Comprehension
- Information
To Demonstrate That:
- Person freely gave consent to participate
- Consent given by a competent person
- Person has been given all information
- Person knows this is research – not treatment
Components of Informed Consent
Must Include the Following Information:
- Why research being done?
- What researchers want to accomplish
- What will be done and for how long
- Risks & benefits of trial
- Other treatments available
- Can withdraw from trial whenever desire
- Compensation for unexpected injuries
Vulnerable Populations
Groups thought not to have autonomy to give informed consent:
- children
- mentally impaired, individuals with dementia
- Prisoners
OR
Who may be unduly influenced to participate:
- students
- subordinates
- pregnant women (actually, the fetuses)
- patients (care-giver vs. researcher)
Inclusion in Clinical Trials
- Historically women were excluded if of reproductive age (ages 18-45)
- Fear of harm to potential unborn child
- In essence, excluded MAJORITY of women
- New guidelines eliminates this stipulation
Participation in Clinical Trials
Why Some Participate:
Give back to society
Exhausted all other txs
Health care services
Payment & incentives
Support
Others?
Why Some Do Not?
- Mistrust of studies
- Do not want to be “guinea pig”
- Do not meet criteria
- Cannot give up time for study visits
- Barriers: lang., distance
Taking Part in Research Studies:
Questions to Ask
- What is study about?
- What are the goals?
- Study sponsor?
- Participant input into protocols?
- Inclusion criteria?
- Benefits & risks
Is there an incentive?
How protected from harm?
What is required: # study visit & what occurs?
What happens after study is over?
How results will be disseminated?
The Impact of Studies
- Some clinical trials have been critical to patient health & provision of health care
- For instance:
Protocol 076: HIV perinatal transmission
1st trial of AZT
Various cancer treatments
Development of other HIV related medications like PIs
Other clinical trials have not been as
successful for a variety of reasons:
- Medications did not work as in laboratory
- Loss to Follow-Up of too many patients
- Harmful substance
- Unethical & poorly conducted study (Ex: Tuskegee Study & recent Gene Replacement Study)
CONCLUSION
- Clinical trails are a valuable part in drug discovery & development.
- Huge costs are involved in conducting clinical trails, so a careful planning is necessary to avoid mistakes and risk involved with clinical trails.
- And a successful trail leads to launch of new drug to people & profits to company.
Thank you for visit and for more pharma updates click here – https://pharmaguidances.com