Warning Letter – October 20, 2023 The U.S. Food and Drug Administration (FDA) inspected your drug manufacturing facility, from February 20 to March 2, 2023. This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations for finished pharmaceuticals. See Title 21 Code of Federal Regulations (CFR), parts 210 and …
Read More »Audit checklist for Purified Water System
Audit checklist for Purified Water System Purified water is water that has been mechanically filtered or processed to remove impurities like bacteria, viruses, chemical pollutants, and minerals like lead and copper, Purified Water is intended for use as an ingredient of official preparations and in tests and assays unless otherwise …
Read More »Good Manufacturing Practices (GMPs)
Good Manufacturing Practices (GMPs) CGMPs provide systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Good Manufacturing Practices (GMPs) are the basic manufacturing and environmental conditions required to produce pharmaceutical products. They ensure that ingredients, products, and packaging materials are handled safely and that pharmaceutical products …
Read More »FDA 483 Warning Letter Dated MARCH 30
FDA 483 Warning Letter Dated MARCH 30, 2022 This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations for finished pharmaceuticals. See Title 21, Code of Federal Regulations (CFR), parts 210 and 211 (21 CFR parts 210 and 211). Because your methods, facilities, or controls for manufacturing, …
Read More »Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance
Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance In various industries, maintaining proper temperature control within critical areas is essential to ensure product quality, integrity, and regulatory compliance. Temperature mapping of storage areas is a systematic process that involves monitoring and analyzing temperature distribution within a defined …
Read More »Equipment in Pharma company as per USFDA
Equipment in Pharma company as per USFDA Equipment design, size, and location (§ 211.63) in Pharma company Equipment used in the manufacture, processing, packing, or holding of a drug product shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its …
Read More »Production and Process Controls in Pharma industry as per USFDA
Production and Process Controls in Pharma industry as per USFDA Written procedures; deviations (21 CFR 211.100). Charge-in of components (21 CFR 211.101). Calculation of yield (21 CFR 211.103). Equipment identification (21 CFR 211.105). Sampling and testing of in-process materials and drug products (21 CFR 211.110). Time limitations on production (21 …
Read More »Definitions as per 21 CFR 210 (USFDA) in Pharma company
Definitions as per 21 CFR 210 (USFDA) in Pharma company (a) The definitions and interpretations contained in section 201 of the act shall be applicable to such terms when used in this part and in Parts 211 through 226 of this chapter for Pharma company. (b) The following definitions of …
Read More »Buildings and Facilities in pharma industry as per USFDA
Buildings and Facilities in pharma industry Design and construction features in pharma industry (a) Any building or buildings used in the manufacture, processing, packing, or holding of a drug product shall be of suitable size, construction, and location to facilitate cleaning, maintenance, and proper operations. (b) Any such building shall have adequate space for the …
Read More »SOP and Guideline for Hold-Time Studies of Tablets
SOP and Guideline for Hold-Time Studies of Tablets These guidelines focus primarily on aspects that should be considered in the design of the hold-time studies during the manufacture of non-sterile solid dosage forms. Many of the principles described here also apply to other dosage forms such as liquids, creams, and …
Read More »All Post URL of Drugs formulations
All Post URL of Drugs formulations Tablets MFR of Albendazole Tablets – (drugsformulations.com) MFR of Alfacalcidol & Calcium Carbonate Tablets (drugsformulations.com) MFR of Sildenafil Citrate Tablets (drugsformulations.com) MFR of Paracetamol & Dicyclomine HCl Tablets – (drugsformulations.com) MFR of Dextromethorphan, Bromhexine, Chlorpheniramine Maleate & Guaifenesin Tablets (drugsformulations.com) Dextromethorphan (drugsformulations.com) Spiramycin Tablet (drugsformulations.com) MFR of Propranolol Hydrochloride …
Read More »Materials in Pharmaceutical as per WHO Guideline
Materials in Pharmaceutical as per WHO Guideline Principle. The main objective of a pharmaceutical plant is to produce finished products for patients’ use from a combination of materials (starting and packaging). Materials include starting materials, packaging materials, gases, solvents, process aids, reagents and labelling materials. No materials used for operations …
Read More »ICH Q8/Q9/Q10 Questions and Answers
ICH Q8/Q9/Q10 1.1 For General Clarification Date of Approval Questions Answers 1.0 June 2009 Is the minimal approach accepted by regulators? Yes. The minimal approach as defined in Q8(R2) (sometimes also called ‘baseline’ or ‘traditional’ approach) is the expectation that is to be achieved for a fully acceptable submission. However, …
Read More »Q7 GMP Guidance for Active Pharmaceutical Ingredients
Guidance for Industry II. QUESTIONS AND ANSWERS A. Scope (1) 1.1: Should GMP according to ICH Q7 be applied for manufacturing steps before the defined API starting material, i.e., steps not identified in grey in Table 1? ICH Q7 does not apply to steps prior to the introduction of the …
Read More »STABILITY TESTING OF NEW DRUG PRODUCTS Q1A(R2)
STABILITY TESTING OF NEW DRUG PRODUCTS Q1A(R2) Drug Product General Photostability Testing Selection of Batches Container Closure System Specification Testing Frequency Storage Conditions Stability Commitment Evaluation Statements/Labeling Drug Product General The design of the formal stability studies for the drug product should be based on knowledge of the behavior and …
Read More »STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2)
STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2) COVER NOTE FOR REVISION OF Q1A(R) The changes made in Q1A(R) that result from adoption of ICH Q1F “Stability Data Package for Registration Applications in Climatic Zones III and IV”. These changes are: The intermediate storage condition has been changed from 30°C ± …
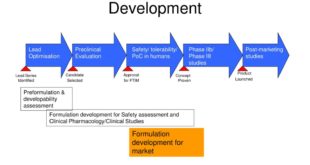
Read More »Pharmaceutical Development as per USFDA
PHARMACEUTICAL DEVELOPMENT I. INTRODUCTION II. PHARMACEUTICAL DEVELOPMENT A. Components of the Drug Product 1. Drug Substance 2. Excipients B. Drug Product 1. Formulation Development 2. Overages 3. Physicochemical and Biological Properties C. Manufacturing Process Development D. Container Closure System E. Microbiological Attributes F. Compatibility III. GLOSSARY INTRODUCTION The Pharmaceutical …
Read More »Cleaning Validation as per APIC Guideline
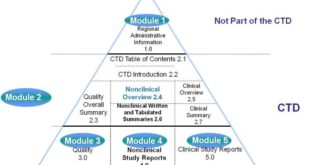
ANDA Submissions — Content and CTD Format (USFDA)
ANDA Submissions — Content and CTD Format (USFDA) TABLE OF CONTENTS CTD FORMAT A. Module 1 – Administrative Information Forms and Cover Letter Administrative Information References Other Correspondence Labeling B.Module 2 – CTD Summaries Quality Overall Summary Clinical Summary C.Module 3 – Quality Drug Substance Drug Product Appendices Regional Information …
Read More »FDA – Warning Letter
FDA – Warning Letter September 10, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more FDA warning Letter …
Read More »