Electronic data logging monitor (EDLM):
A small portable device that measures and stores temperature readings at predetermined time intervals by means of
an electronic sensor. They have programmable alarm capabilities, and integrated displays, and can create reports and graphs which may be permanently stored, shared, and analyzed via proprietary hardware, software, desktop application, or hosted databases.
Installation qualification (IQ):
The process of obtaining and documenting evidence that the premises, equipment, and supporting systems have been provided and installed in compliance with their design specifications.
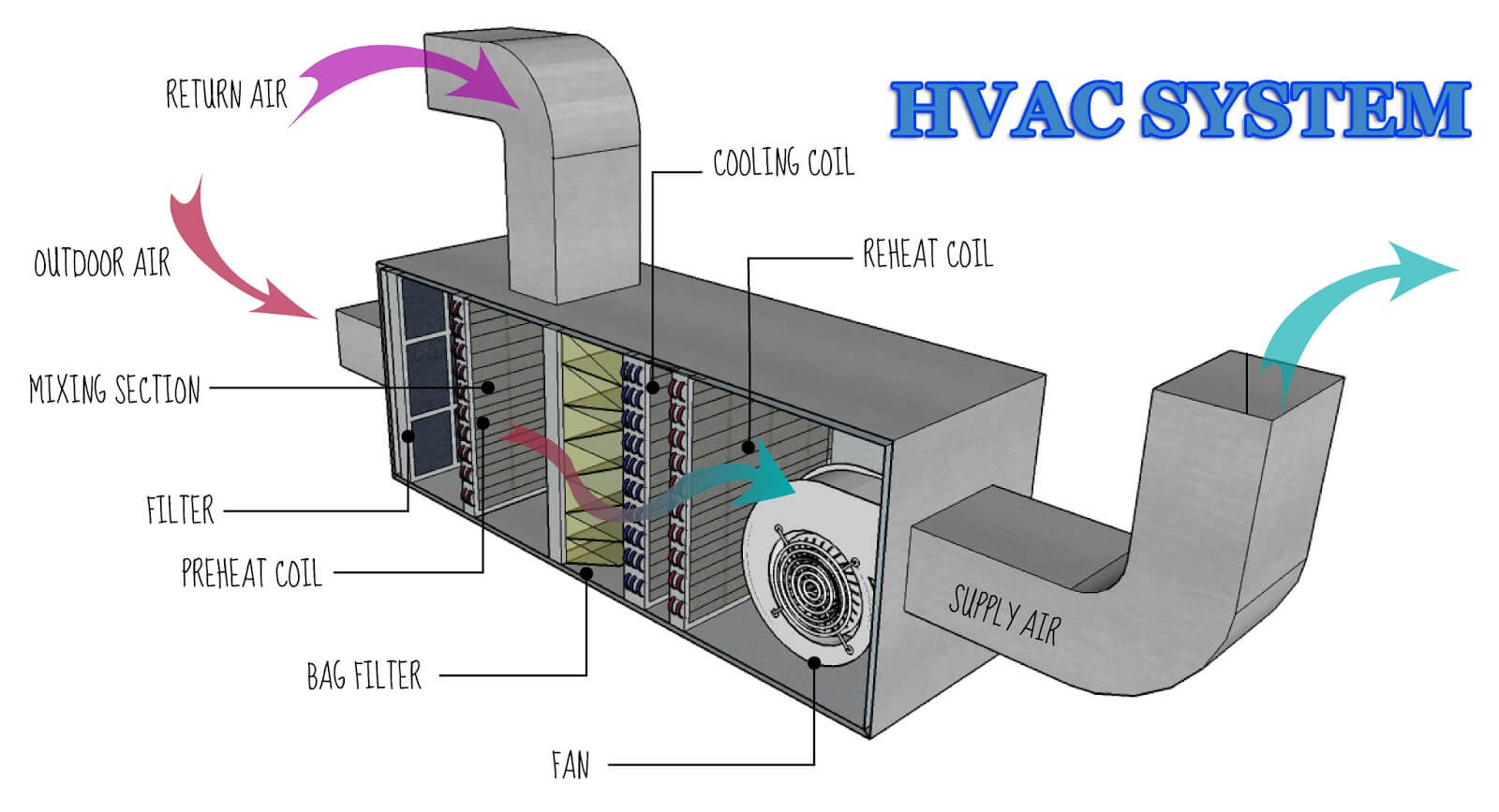
Instrument:
A device that interprets a mechanical, digital, or analog signal generated by a sensor, and converts it into engineering units (°C, percentage relative humidity, mA, etc.) through scaling.
Key operating parameters:
parameters that must be maintained in order to process or produce products with consistent quality attributes and those that may have an impact on the proper operation of the equipment.
Main equipment:
Major equipment to be qualified.
Mapping:
Documented measurement of the temperature and/or relative humidity distribution within a storage area, including identification of hot and cold spots.
Operational qualification (OQ):
The process of obtaining and documenting evidence, under controlled conditions, that the premises, equipment and
supporting systems operate in accordance with their design specifications.
Performance qualification (PQ):
The process of obtaining and documenting evidence that the premises, equipment, and supporting systems, as connected together, will consistently perform in accordance with the approved process
method and specifications.
Pharmaceutical product:
Any product intended for human use or veterinary product intended for administration to food-producing animals, presented in its finished dosage form, that is subject to control by pharmaceutical legislation in either the exporting or the importing state and includes products for which a prescription is required, products which may be sold to patients without a prescription, biologicals, and vaccines. Medical devices are not included.
Refrigeration equipment:
The term “refrigeration” or “refrigeration equipment” means any equipment whose purpose is to lower air and product temperatures and/or to control relative humidity.
Sensor:
A mechanical device (pressure switch, bimetal temperature switch, etc.), or a digital or analog transducer (limit switch, pressure sensor, temperature sensor, etc.) that generates a mechanical or electrical signal to an instrument or
a controller in order to be interpreted.
Service level agreement (SLA):
A service level agreement or contract is a negotiated agreement between the customer and service provider that defines the common understanding about materials or service quality specifications, responsibilities, guarantees, and communication mechanisms. It can either be legally binding or an informal agreement. The SLA may also specify the target and minimum level performance, operation, or other service attributes.
Standard operating procedure (SOP):
A set of instructions having the force of a directive, covering those features of operations that lend themselves to
a definite or standardized procedure without loss of effectiveness. Standard operating policies and procedures can be effective catalysts to drive performance improvement and improve organizational results.
Storage temperature:
The temperature range is listed on the TTSPP label, and within the regulatory filings, for long-term storage.
Temperature-controlled:
Includes any environment in which the temperature is actively or passively controlled at a level different from that of the surrounding environment within precise predefined limits.
Time and temperature-sensitive pharmaceutical product (TTSPP):
Any pharmaceutical good or product which, when not stored or transported within predefined environmental conditions and/or within predefined time limits, is degraded to the extent that it no longer performs as originally intended.
Validation:
Documented testing performed under highly controlled conditions, demonstrating that processes, methods, and systems consistently produce results meeting predetermined acceptance criteria
Reference: Temperature mapping of storage areas (WHO Technical Report Series, No. 961, 2011, Annex 9)