PROCESS VALIDATION PROTOCOL TACROLIMUS CAPSULES Tacrolimus is administered in conjunction with other medications to inhibit the rejection of a kidney, heart, liver, or lung transplant. This particular medication falls under the category of immunosuppressants, which function by diminishing the efficacy of your body’s immune system. Consequently, this aids in the …
Read More »Hydrochlorothiazide and it mechanism of action
Hydrochlorothiazide and its Mechanism of Action Hydrochlorothiazide is a thiazide diuretic used to treat edema associated with a number of conditions, and hypertension. Hydrochlorothiazide is the most commonly prescribed thiazide diuretic. The drug has been widely used to treat hypertension globally and is relatively very safe. Hydrochlorothiazide acts on the …
Read More »CEPHALOSPORINS & MECHANISM OF ACTION
CEPHALOSPORINS & MECHANISM OF ACTION Cephalosporins are a group of semisynthetic antibiotics derived from ‘cephalosporin-C’ obtained from a fungus Cephalosporium. Cephalosporins are chemically related to penicillins the nucleus consists of a β-lactam ring fused to a dihydrothiazine ring, (7-aminocephalosporanic acid). By the addition of different side chains at position 7 …
Read More »SOP On SOP – Initiation of new SOP In Pharma Industry
SOP On SOP – Initiation of new SOP In Pharma Industry The fundamental purpose of an SOP in pharma is to provide clear and concise instructions for the consistent execution of routine operations within an organization. By offering a step-by-step guide on how tasks should be carried out, SOPs aim …
Read More »REGULATORY IMPACT ON OOS
REGULATORY IMPACT ON OOS Stability study required OOS should be reported to RA OOS batch should not be sold to the Regulatory market OOS batch can not be blended with a fresh approved batch OOS batch can not be directly sold to the market Reporting Test Result Result: Averaging Appropriate …
Read More »OOS RECORDING PROCEDURE
OOS RECORDING PROCEDURE REPORTING TO THE LABORATORY SUPERVISOR RECORDING AND NUMBERING OF OOS INVESTIGATION BY ANALYST LABORATORY TESTING APPROVAL BY LAB. SUPERVISOR TESTING BY ANALYST PROCEDURE OF OOS INVESTIGATION: A written record of the review should include the following information A clear statement of the reason for the investigation. A …
Read More »SECONDARY WORKING STANDARDS
REGULATORY RECOGNITION OF SECONDARY WORKING STANDARDS (1) Human Drug CGMP Notes, Vol 9, Number 3, 2001 (Internal FDA Publication): Q: Can a company use reference standards from sources other than the USP? A: Yes. Using a source other than USP can be acceptable provided the reference standard incorporates the critical …
Read More »Temperature mapping protocol
Temperature mapping protocol A well-designed protocol will help ensure that the mapping study is correctly carried out. To cover the full range of temperature regimes, a standard protocol can be used to map any storage area in the facility The mapping protocol should contain the following sections: a. Approval page …
Read More »Temperature Mapping report template
Temperature Mapping report template The mapping report should include the following sections: a. Introduction: a description of the objectives of the mapping study. b. Summary: a summary and discussion of the results organized in the sequence set out in the mapping protocol, including a summary of deviations (if any). c. …
Read More »Electronic data logging monitor (EDLM)
Electronic data logging monitor (EDLM): A small portable device that measures and stores temperature readings at predetermined time intervals by means of an electronic sensor. They have programmable alarm capabilities, and integrated displays, and can create reports and graphs which may be permanently stored, shared, and analyzed via proprietary hardware, …
Read More »GMP Inspection HPLC Checklist
GMP Inspection HPLC Checklist HPLC is an abbreviation for High-Performance Liquid Chromatography. “Chromatography” is a technique for separation, “chromatogram” is the result of chromatography, and “chromatograph” is the instrument used to conduct chromatography. HPLC dedicated to molecular separation called columns and high-performance pumps for delivering solvent at a stable flow rate …
Read More »Audit checklist for Purified Water System
Audit checklist for Purified Water System Purified water is water that has been mechanically filtered or processed to remove impurities like bacteria, viruses, chemical pollutants, and minerals like lead and copper, Purified Water is intended for use as an ingredient of official preparations and in tests and assays unless otherwise …
Read More »Good Manufacturing Practices (GMPs)
Good Manufacturing Practices (GMPs) CGMPs provide systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Good Manufacturing Practices (GMPs) are the basic manufacturing and environmental conditions required to produce pharmaceutical products. They ensure that ingredients, products, and packaging materials are handled safely and that pharmaceutical products …
Read More »Liquid pharmaceutical Preparations
Liquid pharmaceutical Preparations A solution is a homogenous one-phase system consisting of two or more components. →solvent + solute • The solvent is that phase in which the dispersion occurs and the solute is that component that is dispersed as small molecules or ions in the solvent. • Liquid preparations: …
Read More »PARENTERAL PREPARATIONS
PARENTERAL PREPARATIONS Parenteral preparations are sterile preparations intended for administration by injection, infusion, or implantation into the human or animal body. Parenteral preparations may require the use of excipients, for example, to make the preparation isotonic with respect to blood, to adjust the pH, to increase solubility, to prevent deterioration …
Read More »Most common types of pharmaceutical contamination
Most common types of pharmaceutical contamination Contamination can lead to objectionable results in the Pharma industry. It can conciliate the safety of patients, staff, and the environment – as well as affect the market. High standards of hygiene are important to maintain, only preventing the bio-burden levels by means of …
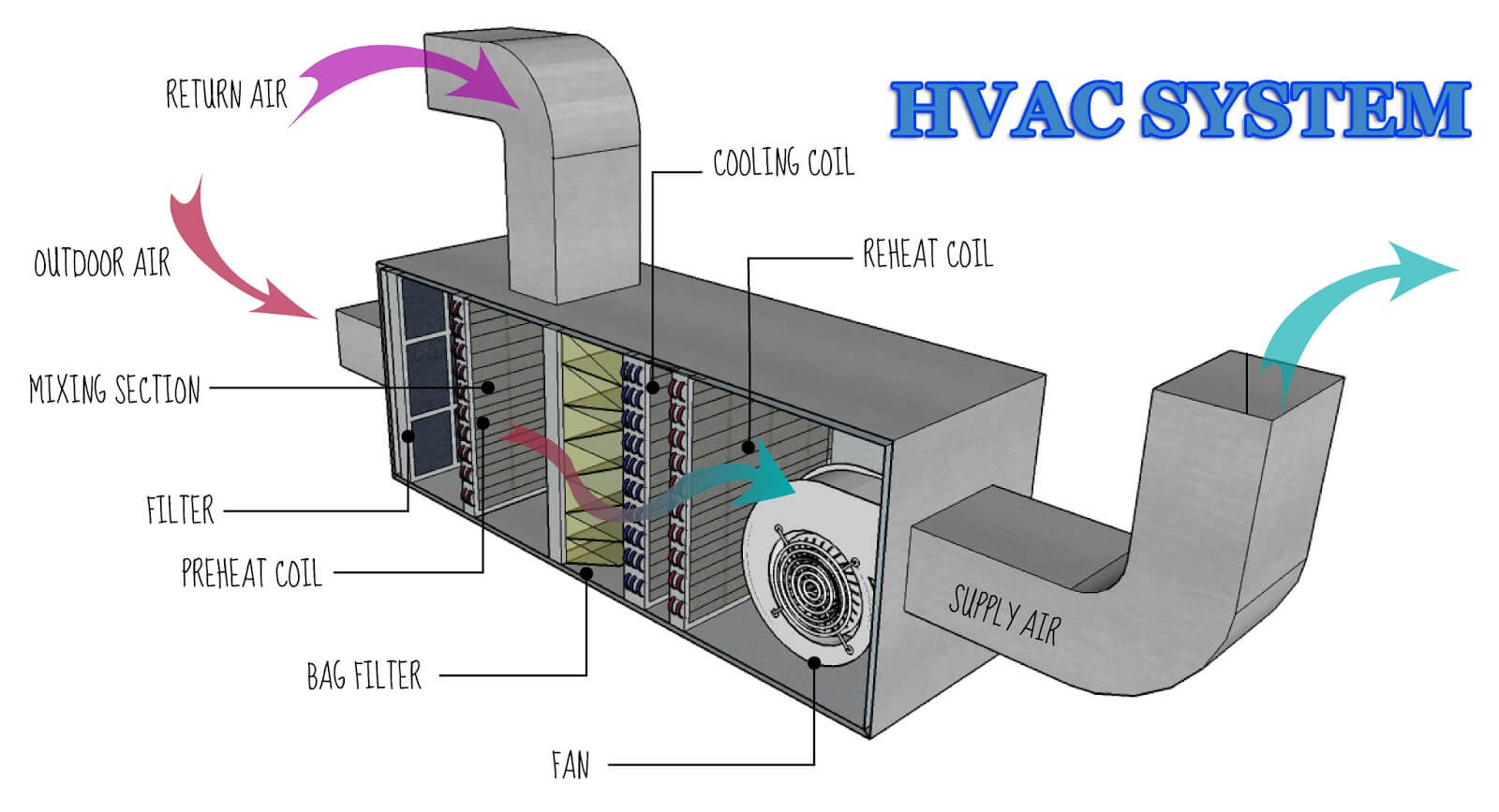
Read More »HVAC SYSTEM AND TEST FOR HVAC QUALIFICATION
HVAC SYSTEM AND TEST FOR HVAC QUALIFICATION What is HVAC? HVAC controls Temperature, Humidity, and Air quality inside a building. HVAC technology aims to provide thermal comfort and acceptable indoor air quality. HVAC system design is a sub-discipline of mechanical engineering, based on the principles of thermodynamics, fluid mechanics, and heat …
Read More »Change Management in Pharma
Change Management In Pharma Change Management in Pharma is a formal process for making planned and unplanned changes to the existing approved procedure and policy. The primary objective of Change Management in Pharma is to provide standardized methods and procedures to meet the change management requirements supporting operations. Change control …
Read More »Hold Time Study Protocol
Hold Time Study Protocol 1. Objective \ Scope 1.1. Hold time study data shall give the assurance of the maximum allowable hold times for bulk and in-process drug products. Generally, one lot can be used for validating hold times if any inconsistency results were observed then another two lots can …
Read More »CRITICAL AND MAJOR DEFICIENCIES
CRITICAL AND MAJOR DEFICIENCIES Compliance and enforcement measures are dependent upon a number of factors, including the significance of violations such as a “Critical” deficiency and a large number of “Major” deficiencies, the history of the site, potential risks to products, and assessment of the manufacturer’s proposed corrective actions. Where …
Read More »