Hold Time Study Protocol
1. Objective \ Scope
1.1. Hold time study data shall give the assurance of the maximum allowable hold times for bulk and in-process drug products. Generally, one lot can be used for validating hold times if any inconsistency results were observed then another two lots can be used for this study.
1.2. Although there are no specific regulations or guidance documents on bulk product hold times, GMP dictates that hold times should be validated to ensure that in-process and bulk products can be held, pending the next processing step, without any adverse effect on the quality of the material. Hold time study provides the re-assurance of the quality at each in-process stage.
1.3. Although there are no specific regulations or guidance documents on bulk product hold times, GMP dictates that hold times should be validated to ensure that in-process and bulk products can be held, pending the next processing step, without any adverse effect on the quality of the material. Hold time study provides the re-assurance of the quality at each in-process stage.
1.4. Although there are no specific regulations or guidance documents on bulk product hold times, GMP dictates that hold times should be validated to ensure that in-process and bulk products can be held, pending the next processing step, without any adverse effect on the quality of the material. Hold time study provides the re-assurance of the quality at each in-process stage.
1.5. Although there are no specific regulations or guidance documents on bulk product hold times, GMP dictates that hold times should be validated to ensure that in-process and bulk products can be held, pending the next processing step, without any adverse effect on the quality of the material. Hold time study provides the re-assurance of the quality at each in-process stage.
2. Responsibilities
2.1. Validation Officer:
- To prepare protocol and report.
- Sampling as per the approved protocol.
- Conclude the result.
2.2. Quality Control:
- Review of Protocol and report.
- To analyze the hold time study samples as per the approved protocol and report the results.
2.3. Quality Control (Microbiology):
- Review of Protocol and report.
- To analyze the hold time study samples as per the approved protocol and report the results.
2.4. Head – Quality Assurance:
- Approval of protocol and result
3. Hold time consideration
3.1. Granulation Solutions, Coating Solutions:
Typically, if these in-process products are used within 24 hours of manufacturing, no bulk holding time studies are deemed necessary. An in-process product that is held for longer than 24 hours should be monitored for physical characteristics and microbial contamination.
A coating solution should be held for the defined hold period. At the test points, a sample should be taken from the storage container and tested. The results obtained should be compared with the initial data of the solution control sample results.
3.2. Powder Blends, Granules:
In-process products such as Powder blends and granules can be held for up to 30 days from the date of production without being retested prior to use. An in-process product that is held for longer than 30 days should be monitored for hold time study under controlled storage conditions for the length of the holding period.
At the test points, a sample should be taken from the storage container and tested. The results obtained should be compared with the initial data of the core tablet and pellet control sample results.
3.3. Core Tablets:
In-process products such as core tablets and extended-release pellets can be held for up to 30 days from the date of production without being retested prior to use. An in-process product that is held for longer than 30 days should be monitored for hold time study under controlled storage conditions for the length of the holding period.
At the test points, a sample should be taken from the storage container and tested. The results obtained should be compared with the initial data of the core tablet and pellet control sample results.
3.4. Bulk Tablets and Capsules:
Bulk tablets and capsules can be held for up to 30 days from the date of production without being retested prior to use. A bulk product that is held for longer than 30 days should be monitored for a hold time study under controlled storage conditions for the length of the holding period.
At the test points, a sample should be taken from the storage container and tested. The results obtained should be compared with the initial data of the tablet and capsule control sample results.
3.5. Oral Liquids and Semi-Solids: (Suspensions, Creams, and Ointments).
Typically, liquid and semi-solid dosage form products should be held for no more than 5 days without a hold time study. Full-scale batches should be used for these studies.
Samples should be taken from the holding vessel after transfer from the manufacturing vessel, and again at the completion of the holding period. Multiple samples should be taken at each time point if holding can impact product uniformity. Samples would be taken to prove the product uniformity of actives and preservatives.
4. Hold time stages
The hold time study for the product shall be carried out in three batches. The validation officer shall collect the sample as per protocol during the manufacturing of the planned batches.
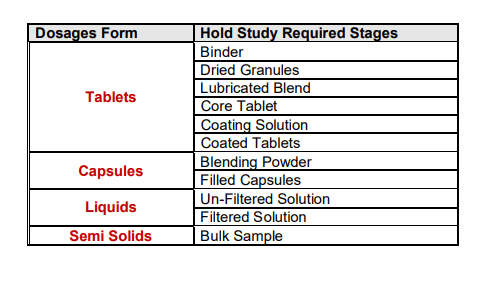
The selection of hold time study conditions is very important for starting the hold study. These conditions are the same as the manufacturing area/hold area conditions, so these conditions are may vary from product to product. Based on the manufacturing process of the dosage forms hold study stages can be decided. Hold study’s required stages are summarized in the table.
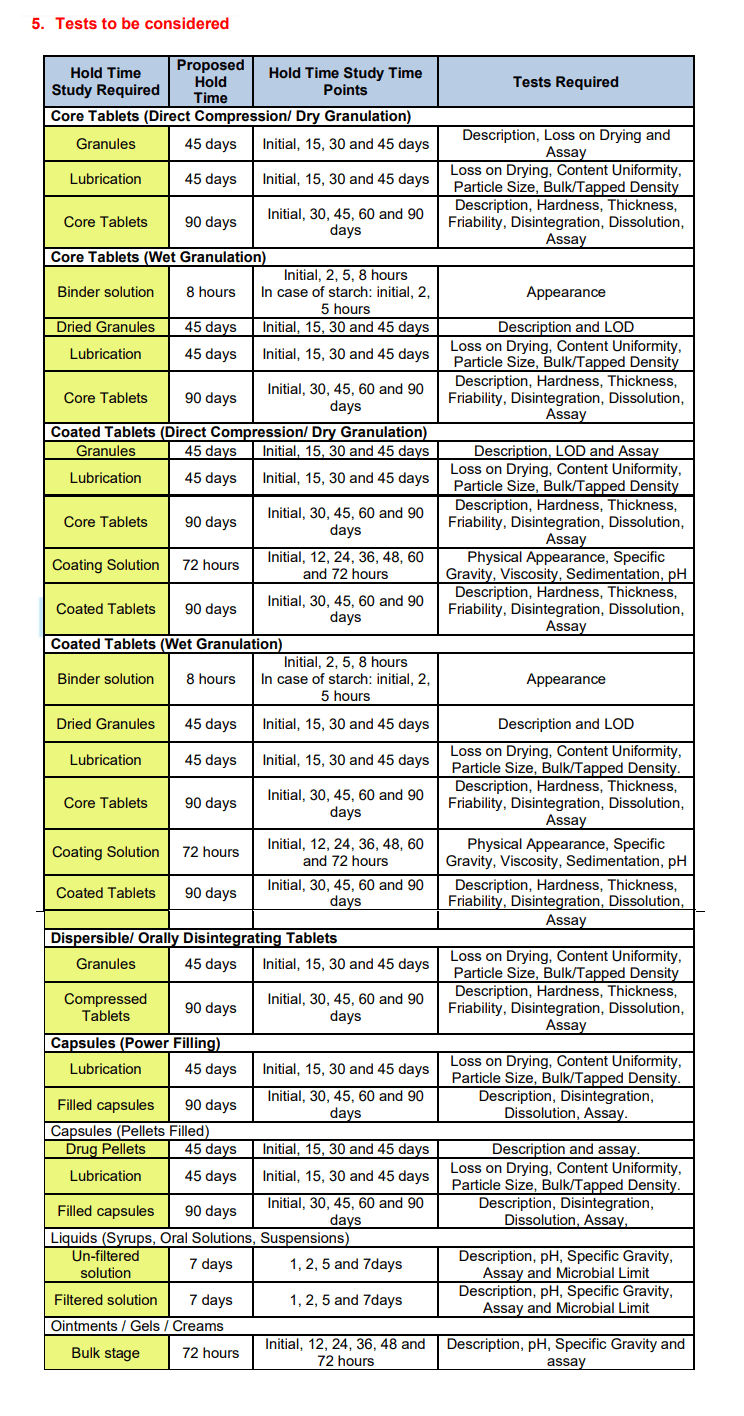
6. Conclusion :
6.1. The conclusion should state whether the outcome of the activity was successful or not.
7. Revalidation Criteria:
7.1. The hold time study shall be performed again in case of any major change in the product specification.

