HVAC SYSTEM AND TEST FOR HVAC QUALIFICATION
What is HVAC?
HVAC controls Temperature, Humidity, and Air quality inside a building. HVAC technology aims to provide thermal comfort and acceptable indoor air quality. HVAC system design is a sub-discipline of mechanical engineering, based on the principles of thermodynamics, fluid mechanics, and heat transfer. The HVAC system is an extremely vital concern, which aids to enhance and maintain the quality of drug products. It mainly helps in achieving optimal temperature, ventilation, and air conditioning in production areas.
The HVAC system design directly impacts the prevention and control of cross-contamination and maintains hygienic conditions at the workplace. Certain pharmaceutical products such as parenteral, bulk drugs,s, etc.
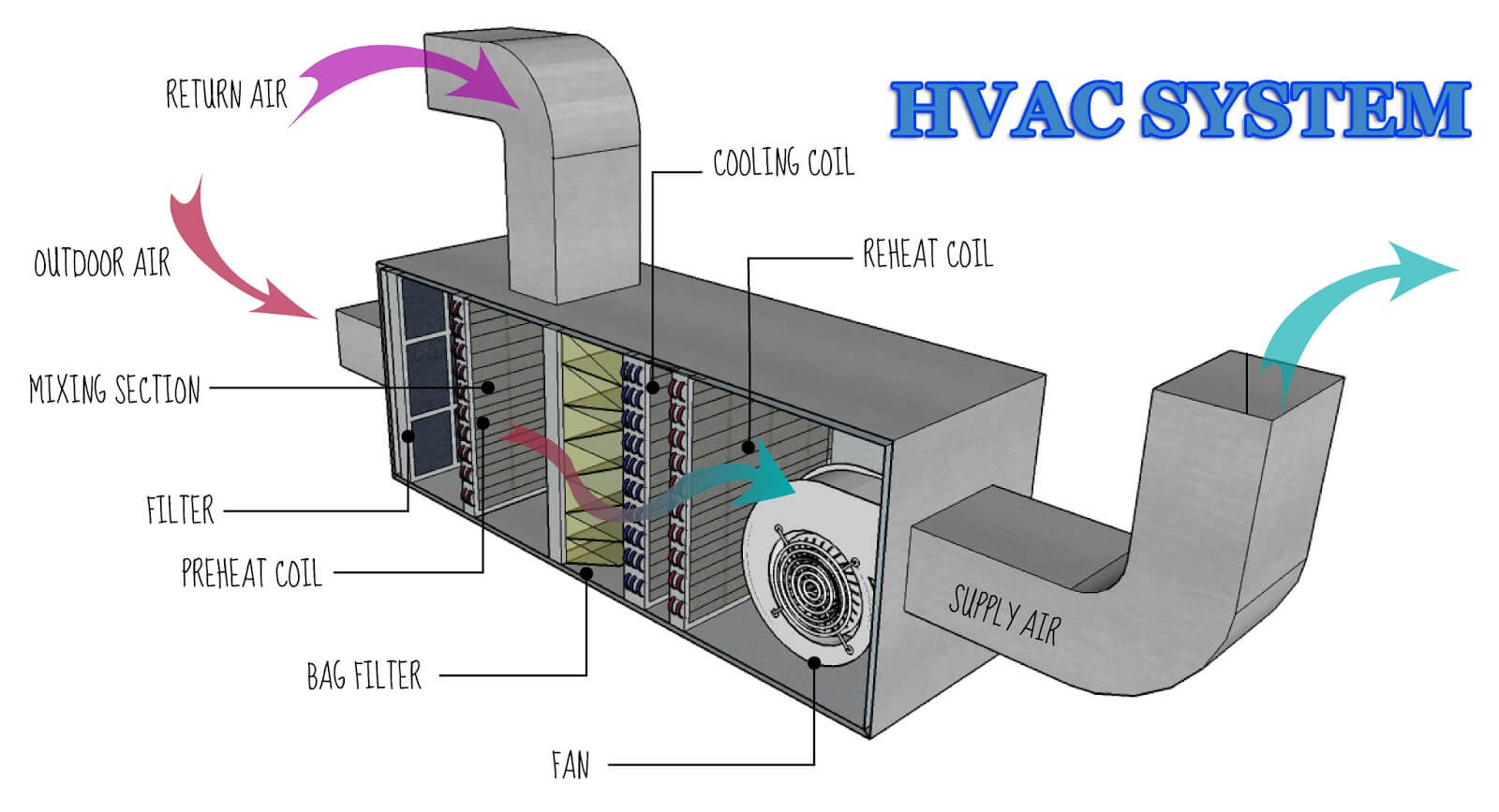
In order to achieve proper cleanliness in the storage and quarantine area, High-efficiency particulate air (HEPA) filters are used. They also maintain the aseptic condition in the working area. The efficiency and integrity of the filters that are used in this system must be checked at regular intervals by performing a leak test.
HEPA filters are the main part of the air handling unit (AHU). The AHU collects the outside fresh air and combines it with the air returning from the cubicles and then supplied the treated air back to the laboratory area. A part of the air exiting from the laboratory rooms is directly exhausted into the atmosphere by an
exhaust fan, while the remaining air is directed to the AHU where it is filtered by passing through prefilters which is attached to the medium filters, to remove any entrapped particles and then the same air is conditioned for humidity and temperature control, and this filtered air is passed to the laboratory and other areas by a supply fan at the desired pressure. HEPA filters are terminal filter which is attached at the entrance to the clean rooms.
BASIC CYCLE OF REFRIGERATION:
Principles of Refrigeration:
- Liquids absorb heat when changed from liquid to gas.
- Gases give off heat when changed from gas to liquid.
Working of Refrigeration Cycle:
- The refrigerant comes into the compressor as a low-pressure gas, it is compressed and then moves out
of the compressor as a high-pressure gas.
ii. The gas then flows to the condenser. Here the gas condenses to a liquid and gives off its heat to the
outside air.
iii. The liquid then moves to the expansion valve under high pressure. This valve restricts the flow of the
fluid and lowers its pressure as it leaves the expansion valve.
iv. The low-pressure liquid then moves to the evaporator, where heat from the inside air is absorbed and
changes from a liquid to a gas.
v. As a hot low-pressure gas, the refrigerant moves to the compressor where the entire cycle is repeated.
| Components of the Refrigeration Cycle | ||
| SNo. | Component | Function |
| 1. | Compressor | Increases the pressure and temperature of the gas by mechanical work done. |
| 2. | Condenser | Change of phase from Gas to Liquid. Heat is rejected to atmosphere from the R-gas. |
| 3. | Expansion Device | Decreases the pressure and temperature of the gas by expansion. |
| 4. | Evaporator | Change of phase from Liquid to Gas. Heat is absorbed by R-gas from the indoor. |
2.1. Compressor:
The function of a compressor is to compress the fluid or gas and increase its pressure and temperature by some mechanical action. Compressors are broadly classified into two types:
i. Positive Displacement Compressors
a. Reciprocating – Single-Acting, Double-Acting, Diaphragm
b. Rotary – Lobe, Liquid Ring, Screw, Scroll, Vane
ii. Dynamic Compressors
a. Centrifugal
b. Axial
Condenser:
Condensation is a process of change of phase from gas form to liquid form, the same process happens inside a
condenser. A condenser converts the refrigerant gas at high pressure & temperature into a liquid refrigerant
at nearly high pressure & temperature by releasing the latent heat from the gas. Condensers are broadly
classified as
i. Air Cooled
ii. Water Cooled
iii. Air and Water Cooled (or) Evaporative Condenser
Expansion Device:
The function of the expansion device is to decrease the pressure & temperature of the liquid refrigerant through the
process of expansion. Types of Expansion Devices:
i. Capillary Tubes
ii. Thermostatic Expansion Valves
iii. Electronic Expansion Valves
Evaporator:
Evaporation is a process of change of phase from liquid to gas form, the same process happens inside an
evaporator. The evaporator converts the liquid refrigerant at low pressure & temperature into its gaseous form
by absorbing the latent heat from the room air. Types of Evaporators:
i. Shell and Tube Evaporator
a. Shell and Tube with the Refrigerant Boiling in Shell
b. Shell and Tube with the Refrigerant Boiling in Tubes
ii. Plate Type Evaporator
Refrigerants:
Refrigerants are the heat transfer media used in the refrigeration cycle, which absorb heat from
the room air during the process of evaporation at a region of low pressure & temperature and releases the
heat during the process of condensation at a region of high pressure & temperature.
Ex: Air, Water, Refrigerants like R-12, R-22, R-134a, R-410a, etc.
CLASSIFICATION OF AIR CONDITIONING SYSTEMS:
Based on the fluid media used in the thermal distribution system, air conditioning systems can be classified as:
- All Air Systems
- All Water Systems
- Air-Water Systems
- Unitary Refrigerant Based Systems
All Air Systems:
In this system, the air is used as the media that transports energy from the conditioned space to the A/C plant. In this system, the air is processed in the A/C plant and this processed air is then conveyed to the conditioned space through insulated ducts using blowers and fans.
It is further classified into 2 sub-systems:
a. Single Duct System – It can provide either cooling or heating using the same duct, but not both
heating /cooling simultaneously.
b. Dual Duct System
All Water Systems:
In this system, the fluid used in the thermal distribution system is water i.e. water transports energy between the
conditioned space and the A/C plant. When cooling is required in the conditioned space then cold water is
circulated between the plant & the conditioned space, while hot water is circulated through the distribution
system when heating is required.
It is further classified into 2 sub-systems:
a. 2 Pipe System – It is used for either cooling or heating-only applications, but cannot be used for
simultaneous cooling and heating.
b. 4 Pipe System – It consists of two supply pipelines – one for cold water and one for hot water, and
two return water pipelines.
Air-Water Systems:
In this system, both air and water are used for providing the required conditions in the conditioned space. The air and water are cooled or heated in a central plant. The air supplied to the conditioned space from the central plant is called primary air, while the water supplied by the plant is called secondary water. The complete system consists of a central plant for cooling or heating water & air, ducting system with fans for conveying air, water pipelines and pumps for conveying water, and a room terminal. The room terminal may be in the form of an FCU, an induction unit, or a radiation panel.
Unitary Refrigerant Systems:
It consists of several separate air conditioning units with individual refrigeration systems. These systems are
factory assembled and tested as per standard specifications and are available in the form of package units of
varying capacities and types. Each package consists of refrigeration and/or heating units with fans, filters,
controls, etc.
HVAC SYSTEM COMPONENTS:
The basic components of a common central HVAC system are:
- Fan(s) to circulate the supply air (SA) and return air (RA).
- Supply air ductwork in which the air flows from the supply fan to the conditioned space.
- Air devices such as supply air outlets and return air inlets.
- Return air path or ductwork in which the air flows back from the conditioned space to the mixed air
chamber (Plenum). - Outside air (OA) device such as an opening, louver or duct to allow for the entrance of outside air into
the mixed air chamber. - Mixed air chamber to receive the return air and mix it with outside air.
- Filter section(s) to remove dirt and dust particles from the mixed air.
- A heat exchanger (s) such as hot water coil(s), steam coil(s), refrigerant evaporator(s), or chilled water
coil(s) to add heat to or remove heat from the circulated air. - Auxiliary heating devices such as natural gas furnace(s) or electric heating element(s).
- Compressor(s) compress the refrigerant vapor and pump the refrigerant around the system.
- Condenser(s) to remove heat from the refrigerant vapor and condense it to a liquid.
- Fan(s) to circulate outside air across air-cooled condenser(s).
- Pump(s) to circulate water through the water-cooled condenser(s); condenser water pump (CWP); and condenser water supply (CWS) and return (CWR).
- Pump(s) to circulate hot water from the boiler(s) through the hot water coil(s) and back or to circulate chilled water from the chiller(s) through the chilled water coil(s) and back to the chiller(s).
- For central systems, water or steam boiler(s) as a central heating source.
- For central systems, water chiller(s) as a central cooling source.
- For central systems, cooling tower(s) with a water-cooled condenser(s).
- Controls to start, stop or regulate the flow of air, water, steam, refrigerant, and electricity.
Components of Air Distribution System:
- Supply Fan
- Coil
- Filter
- Transition fitting
- Supply Air Grill
- Return Air Grill
- Supply Air Duct
- Return Air Duct
- Fresh Air Duct
- Supply Air Diffuser
- Return Air Diffuser
TYPE OF TEST FOR HVAC QUALIFICATION:
- Air Velocity / Air Changes
- Integrity Test of HEPA Filters
- Airborne non-viable particle monitoring.
- Recovery test
- Smoke test (airflow direction)
- Pressure differential, temperature, and relative humidity test
- Airborne viable particle monitoring by settle plate (Passive Air Sampling)
- Airborne viable particle monitoring by air sample (Active Air Sampling)
- Measurement of sound level
Air Velocity / Air Changes:
Objective:
To verify that the HVAC system is capable of delivering the required airflow velocities, and airflow volumes and providing the required number of air changes.
Instruments:
Digital Anemometer, Air Capture Hood.
Procedure:
i. Switch “ON” the air-handling unit.
ii. Put ‘ON’ the anemometer and allow some time for the warming up of the instrument.
iii. Keep the anemometer range as 20-2500 FPM or 0.1-12.7 m/sec.
iv. Remove the filter grill and keep the probe at approximately 150 mm below the face of the filter and velocity recordings shall be noted.
v. Each filter requires a minimum of 5 test spots. Measure the air velocity at 4 corners and one center of the
filter.
vi. Measurement should be taken for a minimum of 15 seconds.
vii. Calculate the air velocity and the number of air changes.
viii. The results shall be recorded in the report.
Acceptance Criteria:
Not less than 20 ACPH
Integrity Test of HEPA Filters:
Objective:
To verify the integrity of HEPA filters
Instruments:
PAO Aerosol generator, Aerosol Photometer
Procedure:
- Filter integrity test shall be performed after the verification of operational velocities and adjustment at
the same. - Supply the compressed air/nitrogen to the Aerosol Generator NLT 2 Kg/cm².
- Introduce the aerosol PAO as a challenge agent into the upstream side of the HEPA
- Switch ON the photometer and select the knob on the upstream side.
- Ensure that the Aerosol concentration in the air stream is 20-80 ug/ lit. Then set the upstream concentration to 100% and close the upstream aerosol port.
- Once the 100% setting is established at the upstream sides turn the instrument knob to downstream.
Keep the scanning probe one inch below the HEPA filter face, and start scanning the HEPA filter face
area and fitment of the HEPA filter with the frame, the speed of scanning of the probe shall be NMT 5 cm/sec.
vii. If any leak is observed then assure that the leakage is coming through the fitment part or HEPA filter
itself. - If fitment leakage is there, tighten the HEPA filter fitment with a pressure plate and it can arrest the leakage. If not arrested then apply silicon sealant to the mounting joint. If leakage is observed through the filter face, the same can be arrested by applying silicon sealant, but the leakage filter face should not exceed 5% of the total filter face. If the leakage area is more than 5% of the total area then replace the filter with a filter as per the new SOP.
- After completion of the activity fix the grill properly to HEPA boxes.
- The results shall be recorded in the respective reports.
Acceptance Criteria:
Leakage should be NMT 0.01%.
Evaluation of result:
i. Results, complying with the acceptance criteria, shall establish the leak of the HEPA filter is acceptable.
If any leakage is observed from the mounting, it has to be rectified through, adjustment and application
of silicone sealant.
ii. Any leakage greater than 0.01% of the upstream challenge aerosol concentration is considered
unacceptable and wants replacement.
iii. If the sealed area is more than 5% of the filter face area /or any individual sealed area is more than 1 in
a filter shall be replaced with a new one as per the defined procedure and qualify the same after
replacement of the filter.
Air borne non-viable particle monitoring:
Objective:
To establish that at different locations within the core process areas, a count of less than specified number of
m or larger is maintained.mparticles per cubic meter of air of 0.5
Instruments:
Laser particle counter
Procedure:
AHU shall be in continuous operation for at least 15 minutes prior to performing this test. Keep the particle
counter ON. Set the particle size channel at 0.5 micron and 5.0 micron. The volume sample at each location
shall be at least 2, 10 liters, with a minimum sampling time of 1 min for grade D and Grade C area respectively
at each predefined location. Select the location number and room ID in particle counter and set it for predefined sampling time then keep the isokinetic probe at working height in the area at predefined location and start the particle counter for taking the particle count, particle count should be done in static condition. Particle count shall be taken at all pre-defined locations number of location shall be calculated by following formula (as per ISO 14644-1).
- Location of particle counts will be based on where the maximum numbers of Non-Viable Particle Counts
are getting highest on specified area such as near riser, equipment positioned man movement and near
entry & exit door. - Calculate the number of sampling point location as per table given below:
Establishment of single sample volume and sampling time per location:
At each sampling location, sample a volume of air sufficient to detect a minimum of 20 particles if the particle
concentration for the largest selected particle size were at the class limit for the designated ISO Class.
Recovery test:
Objective:
To determine whether the core process area is capable of returning to its reference specified class within a
finite time.
Instruments:
Laser particle counter, Aerosol generator
Procedure:
i. Take the initial reading of the area (at working height) for enumeration of particles of > 0.5 µ and 5.0 μ
particles.
ii. Generates the particles in the area with the help of aerosol generator, start the particle count monitoring
until the particles shall reach more than the specified class.
iii. Stop generating particles through aerosol generator once the area reaches to more than approx. 100
times of specified class/initial count.
iv. Take the sample at an interval of 1 minutes and it shall be continued till the desired level of cleanliness
(approx. initial particle count reading or class limit) is achieve.
Acceptance Criteria:
The Recovery time (or decontamination time) shall be not more than 15 minutes.
Smoke test (air flow direction):
Objective:
To visualize airflow pattern of process area operation and to prove that there is no cross contamination from
one area to the other.
Instruments:
Camera to record the airflow pattern of smoke generator
Procedure:
i. Take solution of Poly Glycol and water or purified water in fogger machine for generating the smoke.
ii. After generating, Smoke flow from supply filter to return riser and high to low pressure w.r.t adjacent
room.
iii. Use video camera for recording the flow of smoke.
Acceptance Criteria:
The flow of air from the filter shall sweep the area and shall be towards return air point. Photography /
videography shall be performed for testing airflow pattern. Airflow pattern shall be from positive to negative
differential pressure area.
Pressure differential, temperature and relative humidity test:
Objective:
To demonstrate the capability of the HVAC System to consistently maintain Differential Pressures (DP),
Temperatures and Relative Humidity in different rooms.
Instruments:
Magnehelic Gauge, Thermometers and Hygrometers
Procedure:
i. Ensure that the Thermo hygrometer, Differential Digital Pressure Gauges or digital gauges are calibrated.
ii. Record the Temperature, RH and Differential Pressure shall be monitored at every 04 hours of interval.
Acceptance Criteria:
The system shall be capable of maintaining a Temperature, RH and Differential Pressure as per specified limits (like Temperature: NMT 27°C, RH: NMT 60% and DP: -5 to -30 Pascal).
Air borne viable particle monitoring by settle plate (Passive Air Sampling):
Objective:
To determine the viable particle levels in environment of controlled area by settle plate.
Instruments:
Media plates
Procedure:
i. Test shall be performed at operation condition by the microbiologist.
ii. Prepare the SCDA and SCA plate and enter in to the respective area as per Entry and Exit procedure.
iii. Expose the plates at various locations as per the settle plate location layout.
iv. The plate exposure shall be carried out for the controlled area for three consecutive days after taking
the particle count.
v. After completion of plate exposure, SCDA Plate incubate at 30-35°C for 48 hours and SCA plate incubate
at 20-25°C for 5 days.
vi. After incubation observe the results and record in the data sheet
Air borne viable particle monitoring by air sample (Active Air Sampling):
Objective:
To determine the viable particle levels in environment of controlled area by Air Sampler
Instruments:
Air Sampler
Procedure:
i. Test shall be performed at operation condition by the microbiologist.
ii. Prepare the SCDA plate and enter in to the respective area as per Entry and Exit procedure.
iii. Place the SCDA plate on air sampler and operate the air sampler as per SOP.
iv. Take sampling volume 1000 liter of air as per sampling plan.
v. After completion of sampling incubate the SCDA plate at 20-25⁰C for 72 hours and further transfer at
30-35⁰C for 48 hours.
vi. After incubation observe the results and record in the test data sheet.
Measurement of sound level:
Objective:
To verify the sound level in different rooms
Instruments:
Calibrated Sound Level meter
Procedure:
Operate the sound level meter as per the SOP. Measure the sound level when there is activity in the
areas and record the in test data sheet.
Acceptance Criteria:
Not more than 80 db.
Conclusion:
Qualification of HVAC system has been carried out as per approved qualification plan. All the equipments and
instruments used for the testing of the performance qualification are calibrated qualified. On assessment of
data it was found that uniformity is observed. It can be concluded that obtained values found meeting the
acceptance criteria specified in the plan. Based on the results of qualification data for HVAC system, it is
concluded that system consistently producing air are meeting its predetermined specifications and quality
attributes. Hence the HVAC system is considered to be qualified and can be routinely used.