QUALIFICATION PROTOCOL FOR AIR COMPRESSOR SYSTEM
Air compressor system -The purpose of carrying out Qualification of Air compressor system is to establish the documented evidence that the system performance is in accordance with the predefined specifications. The scope of this protocol is to describe the procedure for different tests, acceptance criteria, Re-validation criteria and documentation to be used in validation of compressed Air system user point. This protocol shall be applicable to all the compressed air and used point
AIR COMPRESSOR SYSTEM
- The main aim of the Air compressor system is to produce and provide the desired quality compressed air as per the designed capacity at designed pressure.
- The Air compressor system and quality of the compressed air distributed to all user points must comply with ISO8573, USP, BP and in-house requirements. The Quality refers to the amount of particles. Humidity and oil content in the air. For solid particles counts of 4,00,000 particles of diameter from 0.1 µ to 0.5 µ. 6000 particles from 0.5 µ to 1µ and 100 particles from 1 µ to 5 µ (applicable to product contact points only) is required. For humidity, a dew point of <40°C is considered and for oil content a limit of 0.01 mg /m3 is considered.
Capacity of Air compressor system
The generation system of compressed air by 3 air compressors having the capacity of 218 CFM, 125 CFM and 125 CFM.
TABLE OF CONTENTS
- RE-APPROVAL
- OBJECTIVE
- SCOPE
- SYSTEM DESCRIPTION
- USE
- OPERATION & DESIGN FEATURE
- Compressed air system:
- PERFORMANCE RE-QUALIFICATION TEST PLAN
- RESPONSIBILITY
- TEST EXECUTION METHOD
- ACCEPTANCE CRITERIA
- SUMMARY REPORT AND CONCLUSION
- ENCLOSED DOCUMENTS
- ABBREVIATIONS
PRE-APPROVAL
This document is prepared by Validation Team under the authority of their QA Manager. Hence, this document before being effective shall be approved by the QA Manager.
OBJECTIVE:
The purpose of carrying out Performance Re-Qualification of Compressed Air system is to establish the documented evidence that the system performance is in accordance with the predefined specifications.
SCOPE
The scope of this protocol is to describe the procedure for different tests, acceptance criteria, Re-validation criteria and documentation to be used in validation of compressed Air system user point. This protocol shall be applicable to all the compressed air and used point.
Below is the detail of Air Compressor:
Name of the System: Compressed Air system.
Supplier Name: Ingersoll rand.
This protocol should be generated to qualify the performance of the system. In case of further modification or relocation, some part of the same protocol can be used or separate protocol or addendum can be generated.
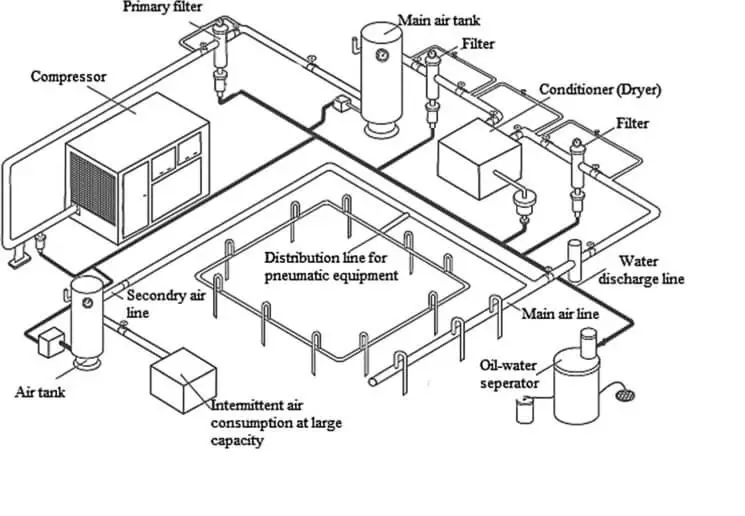
COMPRESSED AIR SYSTEM DESCRIPTION
USE OF COMPRESSED AIR SYSTEM
- The main aim of the system is to produce and provide the desired quality compressed air as per the designed capacity at designed pressure.
- The Compressed Air System and quality of the compressed air distributed to all user points must comply with ISO8573, USP, BP and in-house requirements. The Quality refers to the amount of particles. Humidity and oil content in the air. For solid particles counts of 4,00,000 particles of diameter from 0.1 µ to 0.5 µ. 6000 particles from 0.5 µ to 1µ and 100 particles from 1 µ to 5 µ (applicable to product contact points only) is required. For humidity, a dew point of <40°C is considered and for oil content a limit of 0.01 mg /m3 is considered.
Capacity
The generation system of compressed air by 3 air compressors having the capacity of 218 CFM, 125 CFM and 125 CFM.
OPERATION & DESIGN FEATURE
Air Compressor system:
The Moisture free Air compressor system comprises of state-of-the-art Vertical single cylinder reciprocating air cooled compressor with “V” Belt arrangement, starter cum control panel, safety devices, interconnecting piping, cabling etc. and accessories duly mounted on a rigid steel deck. These packages are provided with anti-vibration mountings to reduce the vibration from being transmitted to the floor hence eliminating elaborate civil foundation.
There is three compressed air generator installed at the ground floor.
The following main parts were considered in the design of the compressed air generator:
Main Frame Body of Air Compressor system: Totally enclosed, rigid pressure tight made of high grade, close grained C.I precision machined and stress relieved for permanent alignment. Two bearing fitted on the drive end side gives better support to the crank shaft aided with one bearing fitted on the pump end. Main bearing housing bore are accurately bored to avoid misalignment.
Crank Shaft of Air Compressor system: High grade S.G Iron crankshaft and journals duly ground and polished ensure a long life of bearings.
Connecting Rods of Air Compressor system: Forged alloy steel connecting rod duly normalized is designed to provide minimum thrust on the crosshead bearings surfaces. Big end bearings bores finish machined accommodate prefinished replaceable bearing halves of copper lead alloy designed for operation. Small end bearing bushings are of special bronze metal.
Main Bearing & BIG End Bearing: thin walled, pre-finished to the size, small width and sufficiently large diameter perfect rigidity to miming gear. Bothe bearings are copper-lead alloy
Cross Head: should be made lush grade S.G. Iron. Its low inertia along with low friction cross-slides ensures perfect miming of cross head. It’s hallow design on either sided ensures true miming of piston rod thereby resulting in improved life of packing and maximum life of piston rod.
Cylinder: Graded C.I cylinders designed with adequate coolant in jacket ensure optimum heat dissipation of the surfaces in contact with compressed air. Wide jacket door provided on cylinders ensure easy access for cleaning of cylinder jackets. Should be designed for streamlined air passage and maximum no. of valves Cylinders provide smooth flow of air thereby minimizing the pressure drop.
Piston: for Low inertia. Light weight, piston in two halves of special aluminum alloy are double acting and provided with suitable compression rings to ensure perfect sealing during compression. In addition to piston rings piston also provided with Teflon rider ring to take load on the piston during unloaded condition
Piston Rod & Packing: alloy steel piston rods fitted with wear resistant packing rings of antifriction type to prevent any possibility of compressed air leakages
Valves: Reduced lift with large flow area, stainless steel, ported plate type valves, both for suction and discharge arranged symmetrically for long life. A protection grill fitted on suction valve prevents any valve part failing into the cylinder in case of any accident. Each of accessibility of these valves of non-reversibility of suction and discharge valves make simpler and fool proof for regular maintenance
Oil Pump : Gear type oil pump directly driven by crankshaft and fitted on the bearing housing. So it feeds oil to the main bearings, connected rod bearings and to cross head slides. The oil pressure is regulated by a pressure regulating screw provided on the oil pump body. To increase the pressure regulating screw has to be screwed in and vice versa.
Receiver of Air Compressor system: MS vertical receiver for storage of compressed air and to drain the moisture content if any. The receiver contains automatic moisture drain trap, safety valve for High air pressure in the receiver.
Air dryer of Air Compressor system : the air entering the system enters into the pre cooler. A pre cooler is a heat exchanger where the incoming hot air is being cooled by the outgoing cold air so as to reduce the heat loads for the evaporator and thereby the refrigeration system. Tins is done with an ultimate aim to reduce the refrigeration compressor power. Normally the moisture will be in the water vapor will get condensed. It is easy to remove the moisture as a condensate than the moisture in water vapor state. For every drop in temperature of air. there will be formation of condensate moisture.
The system has the provision to indicate, monitor, the parameters of Oil pressure, Air temp. Discharge air pressure.
The system should have the provision enabling the loading and unloading system depending on the consumption conditions in the process operation.
The system should have the necessary connections for the cooling system, safety system for the smooth operation of equipment.
QUALIFICATION TEST PLAN FOR AIR COMPRESSOR SYSTEM
Qualification test of Air compressor system shall include following test:
Qualification Test which contains following test
| Test Name |
| Non-Viable particle count |
| Viable particle count |
| Moisture content of Air |
| Oil and Grease test |
| Medical Air test for (CO + CO2, NO + NO2, SO2,) Contest |
| Hydrocarbon |
| Dew Point |
Verification of measures identified during risk analysis
All related Operation related requirements as identify in the risk assessment are verified for compliance.
TEST METHODOLOGY
Qualification tests shall be performed for 03 days. Or 03 consecutive time as per sampling point given in Annexure-1. All the tests mentioned as per data sheet 3 shall be be carried out at critical points
TEST EXECUTION METHOD
PRE –REQUISITES
Prior to conducting/ executing the Performance Re-Qualification protocol following conditions must be fulfilled:
- Air compressor system should be safe for execution.
- Approval of Qualification report
SIGNATURE REGISTRATION & TRAINING
All personnel who are executing or reviewing the protocol must enter his/her name and signature in signature registration page. Provide the location of training record or attach the appropriate training record with the report to indicate that the personnel should be trained on the following:
- Execution of RQ protocol
- Writing GMP critical record
- Deviation handling procedure for validation related deviations
- Review of executed validation protocol and GMP critical records
GENERAL RECORDING INSTRUCTION
- Execution will be carried out as per the SOP “Procedure for Qualification/ Requalification Procedure for System/Equipment/ Utility/ Instruments”.
- Recording of observation will follow good documentation practice..
- Recording will be done on a controlled copy of the approved protocol issued by Quality Assurance.
- In the test data sheet test parameter and criteria will be pre-defined. Other cells e.g. observation and signature will be completed by the person manually.
- Where observation is to be recorded as ‘Y/N/NA’, write yes when the observation is in compliance with acceptance criteria, write No when observation is non-compliance. If it is not applicable write NA, if unobvious write suitable justification for being not applicable
- Any mistake in the approved protocol format if identified before or during execution shall be recorded as comment rather canceling it manually. This mistake will be verified during review of executed protocol.
- Comments and deviation will be recorded as per the instruction given in the following section.
- If possible or required a digital photograph may be taken and print of the photograph may be presented as evidence of compliance or deviation from the acceptance criteria.
DEVIATION HANDLING
- During execution the comments if any will be noted in the respective datasheet.
- All comments shall be numbered as “X-YY” where “X” is test sheet no. and “YY” is the sequential serial no. for that particular test sheet; For example in test sheet no. 3, second comment shall be numbered as 3-02. Comment number shall be allotted on the test data sheet and comments shall be written on comment summary sheet.
- During review or execution all comments will be verified and if any comment is made to specify non-compliance to that test acceptance criteria, comment will be escalated as “Deviation”.
- The deviation will be identified and it will be suitably numbered in the comment section of the respective datasheet.
- The deviation will be assessed based on its GMP criticality. GMP non-critical deviations can be justified whereas GMP critical deviation may require investigation and corrective actions. Appropriate justification, investigation, corrective action and verification of effectiveness of corrective action will be recorded in the deviation data sheet # 5. Comment summary will be recorded in the deviation data sheet # 6.
GENERAL SAFETY INSTRUCTION FOR EXECUTION
Safety will be one of the key considerations during the execution of this protocol. The following guidelines must be observed during the execution stage.
- All personnel involved with the execution shall identify hazards associated with performance of PQ testing and precautions to be taken.
- All personnel involved with the execution shall inform to company management any hazard, to themselves or others, associated with the materials, equipment, method of working and the precautions to be taken.
- All personnel involved with the execution shall check that utilities are safely isolated when energizing or de-energizing
ACCEPTANCE CRITERIA
The system successfully passes RQ if all the test specifications are passed or open tests justified and accepted.
Thus it is shown that the system
- Meets the Specifications and Quality requirements identified by the User.
- Is correctly performed and documented.
RE-QUALIFICATION CRITERIA:
1 Scheduled Requalification.
2 Revalidate the equipment in the following cases.
- Change of location.
- Break down of Critical parts.
SUMMARY REPORT AND CONCLUSION
In order to close the Qualification of Air compressor, the tests results shall be evaluated and the qualification report (format enclosed) shall be formally approved. During the review of the report it is necessary to assess to what extent all tests were successfully completed. GMP critical deviations must be completely fulfilled before routine production activity.
(A) Sampling Plan
| S. No | Room Name /No. | Test | ||||||||||||||||||||||||||
| Oil content Test | Non Viable Particle | Viable particle count test | Moisture Content | CO2 | NO+NO2 | CO | SO2 | Hydrocarbon | ||||||||||||||||||||
| Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
Day
1 |
Day
2 |
Day
3 |
||
| 1 | FFS Machine | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2 | Cooling Zone (for Integrity Test Apparatus | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3 | Super-Heated Water Spray Sterilizer (SHWSS) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4 | Disinfectant & Media Plates Prep. Room (Micro lab-II) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5 | Air Receiver Tank | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 6 | Uflex Powder Filling Machine (ORS) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 7 | Solvent Room | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 8 | Coating Area | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 9 | Service Floor-I (Filter Cleaning Room) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 10 | Service Floor-II (Filter Cleaning Room) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
Remark: The rationale behind selecting the above sampling points is based on the risk assessment of all the sampling points and selecting the worst case scenario depending on the following two factors: Impact Analysis: The compressed air user points could be broadly classified into following 3 classes:
- Direct impact – These are the points where the compressed air comes in direct contact of the product during manufacturing process.
- Indirect impact – These are the points where the compressed air is not in direct contact with the product, but is rather used for drying of the manufacturing equipment’s after CIP/ WIP.
- No impact – These are the user points where the compressed air is used only for pneumatic operations and hence have no effect on the product quality.
All the direct & indirect impact user points are installed with a 0.2µm filter at the point of use. Since the indirect impact user points do not directly affect the product quality, hence the 0.2 µm terminal filter is sufficient to meet the require acceptance criteria.
However, for the direct impact user points testing of all the parameters are required, as any failure might have a direct impact on the product quality. In the current facility, as the compressed air is in direct contact of product in only two equipment’s i.e. RMG and Be-coater, hence only two user points have been selected for the testing of the complete compressed air quality. These two user points are situated in the Granulation and Coating room, respectively.
- Distribution system length: The length of the distribution system plays an important part in deciding the physical quality parameters of the compressed air, hence it is suggested to sample the last user point of the distribution loop as that would simulate the worst case scenario. Therefore, the user point at Packing hall has been selected for testing. In addition, the quality of the air generated by the compressor before passing through distribution lines also needs to first qualify on all the parameters, hence the initial point after generation have also been considered in the sampling plan.
- As this is the initial phase of testing therefore to confirm the parameters complying with the limits as mentioned in ISO 8573-1, all the test are marked for all the sampling points. However, after establishment of evidence of conformance, testing can be performed only at first and last sampling points i.e. Generation and Packing hall, respectively.
Acceptance criteria:
| Moisture content# | Oil content* | Non Viable Particle Count*
(Maximum number of particles per m3) Particle size, d |
Microbial Limit** | ||
| 0.1 < d ≤ 0.5 µm | 0.5 < d ≤ 1.0 µm | 1.0 < d ≤ 5.0 µm | |||
| Not more than 67 PPM | ≤ 0.1 mg / m3 | NMT 20000 | NMT 400 | NMT 10 | ≤ 200 CFU / m3 |
| CO2* | NO+NO2* | CO* | SO2* | Hydrocarbon |
| NMT 500 ppm | NMT 2 ppm | NMT 5 ppm | NMT 1 ppm | NMT 1 ppm |
Verification of measures identified in Risk Assessment
| Acceptance Criteria | RA ref no | Compliance | Document Reference
(if applicable) |
Comment Ref No | |
| Yes/No/ NA | Document Name/type | Document no &/or location | |||
| Air quality according to EP, and USP and in-house requirement (particle size, – residual oil 0, 1mg/m3, dew point & water content. | 8 (20) | ||||