GMP Inspection HPLC Checklist
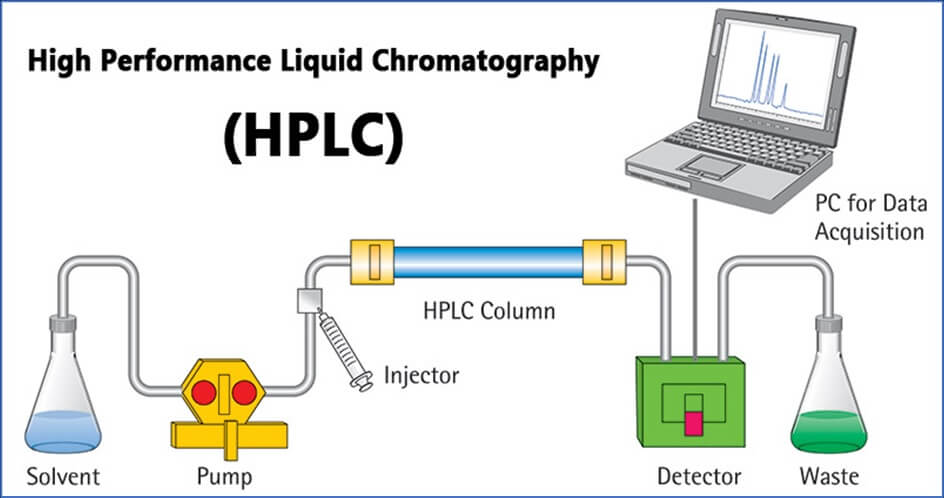
HPLC is an abbreviation for High-Performance Liquid Chromatography. “Chromatography” is a technique for separation, “chromatogram” is the result of chromatography, and “chromatograph” is the instrument used to conduct chromatography. HPLC dedicated to molecular separation called columns and high-performance pumps for delivering solvent at a stable flow rate are some of the key components of chromatographs. Only compounds dissolved in solvents can be analyzed with HPLC. HPLC separates compounds dissolved in a liquid sample and allows qualitative and quantitative analysis of what components and how much of each component is contained in the sample.
Chromatographers can ensure the accuracy, reliability, and efficiency of their analyses by HPLC checklist. A meticulous approach to each step of the HPLC process contributes to the generation of high-quality chromatographic data, supporting research, quality control, and various applications across industries. Regular maintenance, adherence to best practices, and attention to detail are key elements in achieving optimal results with HPLC
A comprehensive HPLC checklist to guide chromatographers in optimizing their analyses and maintaining the integrity of their results as follows
1. Preparation Phase:
a. Sample Preparation:
Ensure samples are properly prepared to avoid contamination or degradation.
Verify that samples are compatible with the chosen HPLC method.
b. Standards and Calibration:
Prepare calibration standards with known concentrations.
Confirm the calibration curve’s linearity and accuracy.
c. Column Selection:
Choose the appropriate column based on the nature of the analytes.
Verify column compatibility with the mobile phase.
2. Instrument Setup:
a. Column Installation:
Properly install and secure the HPLC column according to manufacturer guidelines.
Check for column integrity and ensure it is free from leaks.
b. Mobile Phase Preparation:
Prepare the mobile phase with high-quality solvents.
Degass the mobile phase to eliminate air bubbles that may affect baseline stability.
c. Instrument Calibration:
Calibrate the HPLC system for accurate retention time and peak area measurements.
Verify pump flow rates and detector sensitivity.
3. System Check:
a. Leak Check:
Perform a thorough check for leaks in the entire system, including tubing, fittings, and the column.
Rectify any identified leaks promptly.
b. Baseline Stability:
Stabilize the baseline by running the mobile phase without sample injection.
Ensure a consistent and noise-free baseline.
c. System Pressure:
Monitor system pressure and check for any irregularities.
Adjust the pressure settings if necessary.
4. Sample Injection:
a. Injection Volume:
Use the appropriate injection volume based on the analytical method.
Avoid overloading the column with excessive samples.
b. Sample Integrity:
Verify that the sample vials are properly sealed to prevent contamination.
Minimize exposure of samples to light and air.
5. Data Acquisition:
a. Detector Settings:
Optimize detector settings for maximum sensitivity and signal-to-noise ratio.
Ensure that the detector wavelength is suitable for the analytes.
b. Data Recording:
Verify that the data acquisition system is recording accurately.
Save raw data and chromatograms for future reference.
6. Post-Analysis:
a. Column Cleaning:
Clean the column regularly according to the manufacturer’s recommendations.
Store the column properly to extend its lifespan.
b. Waste Disposal:
Dispose of waste solvents and materials responsibly.
Adhere to environmental and safety regulations.
c. Documentation:
Document all steps and parameters, including instrument settings and deviations.
Maintain a detailed log for traceability and troubleshooting.
HPLC Related Most Frequently Asked Questions
Do you have policies and procedures that specify how the staff is given access to HPLC systems?
Are individual login and passwords required to access HPLC systems?
Does your software control actions through access privilege levels?
Are the date and time featured secured so they cannot be changed by those who perform, supervise, or review records?
Is the identity of the person performing an activity captured by the audit trail?
Are your audit trails capable of documenting why an action was performed?
Is the data being reviewed in the same format in which it was generated?
Is the mobile phase prepared as per STP?
Is the mobile phase valid for a recommended date?
Check the calibration status of the instrument
Is the column available as per standard testing procedure?
Is the entry of the column in the logbook maintained?
Is the right method selected by the user?
Is the sequence of the sample set as per STP?
Is the flow rate of the method as per STP?
Is the sample prepared as per STP?
Is the system suitability pass as per STP?
Are no fluctuations in column pressure?
Is no leakage observed in the system?
Are all peaks integrated properly?
is the RSD of the area within the limit?
Is bracketing of injection within the limit?