HPLC Chromatography
High-Performance Liquid Chromatography (HPLC) has revolutionized the field of analytical chemistry, offering a powerful and versatile tool for separating, identifying, and quantifying compounds in complex mixtures. High-Performance Liquid Chromatography has become an indispensable tool in various scientific disciplines, contributing significantly to advancements in research, quality control, and diagnostics. Its versatility, sensitivity, and ability to handle complex samples make it a cornerstone in the analytical arsenal of scientists and researchers worldwide. As technology continues to evolve, so too will the capabilities and applications of HPLC, ensuring its continued prominence in the realm of analytical chemistry.
Applications of HPLC:
1. Pharmaceutical Analysis: HPLC plays a crucial role in pharmaceutical research and quality control, enabling the analysis of drug formulations, impurities, and degradation products.
2. Environmental Monitoring: HPLC is employed for the detection and quantification of environmental pollutants, such as pesticides, herbicides, and heavy metals in air, water, and soil samples.
3. Food and Beverage Industry: HPLC is utilized for the analysis of food and beverage products, ensuring the safety and quality of consumables by detecting contaminants and additives.
4. Clinical Diagnostics: In clinical laboratories, HPLC is used to analyze biological samples for the presence of drugs, metabolites, and other biomolecules, aiding in disease diagnosis and monitoring.
5. Biochemical Research: HPLC is a valuable tool in biochemistry for separating and analyzing complex mixtures of proteins, peptides, and nucleic acids.
HPLC Chromatography troubleshooting
There is no standard troubleshooting procedure.
General Pattern:
- Locate the problem by ranking possible causes.
- Verify the presence of the most probable cause.
- If present – fix the problem, otherwise verify the existence of the next possible cause.
Two Types of HPLC troubleshooting System problems or Method problems
HPLC System Components
•Pump
•Injector/ Autosampler
•Column
•Detector
•Data System/Integrator
Problems Can be related to all components in the system
Method vs. System Troubleshooting
System Parameters
• Flow stability
• Backpressure
• Clogging
• Detector problems
• Injection suitability
Method Parameters
• Flow rate
• Eluent composition
• pH &pH modifier (type)
• Injection volume
• Temperature
• Gradient profile
System Parameters
Simple preliminary verification of system setup can save time.
| Solvent | Degasser | Pump | Autosampler | Column | Detector |
| Bottle fill-in Inlet filter date | Flush if solvent change >15 mL | Backpressures Flow stability Check-valves | Vial fill-in connections cross[1]contamination | Column type connections | Wavelength |
Critical connections. Minimize tubing length
Categories of Column and System Problems
I. Pressure
II. Peak shape
III. Retention
IV. Detection
I. Pressure Issues
| Column observations | Potential Problems |
| High pressure | – Plugged frit – Column contamination – Plugged packing |
| Low Pressure | – Leak – Flow Incorrect |
Determining the Cause and Correcting High Back Pressure
Many pressure problems are due to blockages in the system.
If Column pressure is high:
• Back flush column – Clear “dirty” frit surface
• Wash column – Eliminate column contamination and plugged packing
➢– high molecular weight/adsorbed compounds
➢– precipitate from sample or buffer
Column Cleaning
Flush with stronger solvents than your mobile phase
Reversed-Phase Solvent Choices in Order to Increasing Strength
➢Mobile phase without buffer salts
➢100% Methanol
➢100% Acetonitrile
➢75% Acetonitrile:25% Isopropanol
➢100% Isopropanol
➢100% Methylene Chloride*
➢100% Hexane*
Use at least 25 mL of each solvent for analytical columns
* When using either Hexane or Methylene Chloride the column must be flushed with Isopropanol before returning to your reversed-phase mobile phase.
Prevention Techniques for column problems
Use column protection
➢In-line filters
➢Guard columns
• Filter samples
• Filter buffered mobile phases
• Sample clean-up (i.e. SPE)
• Appropriate column flushing
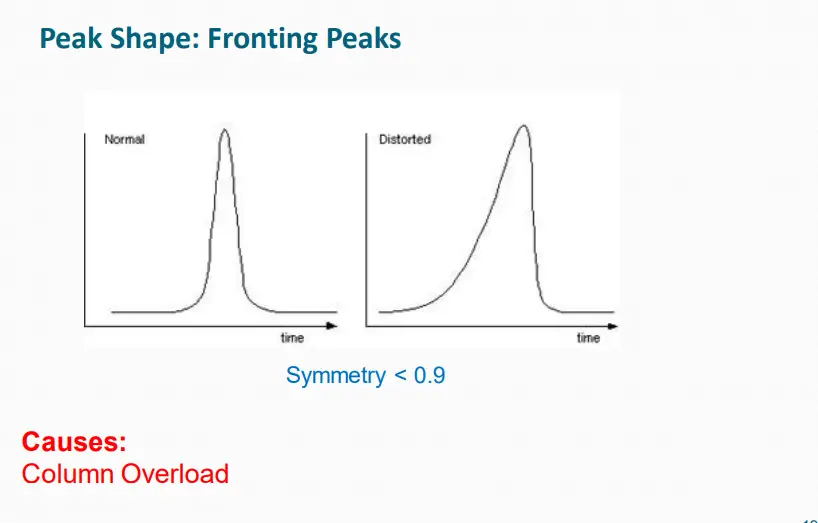
II. Peak Shape Issue
What Are Common Peak Shape Issues?
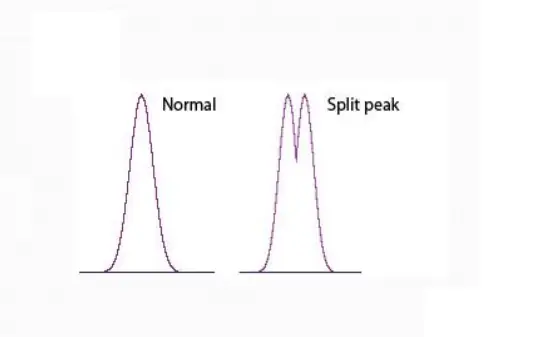
1. Split peaks
2. Peak tailing
3. Broad peaks
• Many peak shape issues are also combinations – i.e. broad and
tailing or tailing with increased retention
•Symptoms do not necessarily affect all peaks in the chromatogram
•Each of these problems can have multiple causes
Peak Splitting Caused By Disrupted Sample Path
•Flow Path Disrupted by Void
•Sample Allowed to Follow Different Paths
Through Column
•Poorly Packed Bed Settles in Use
•High pH Dissolves Silica
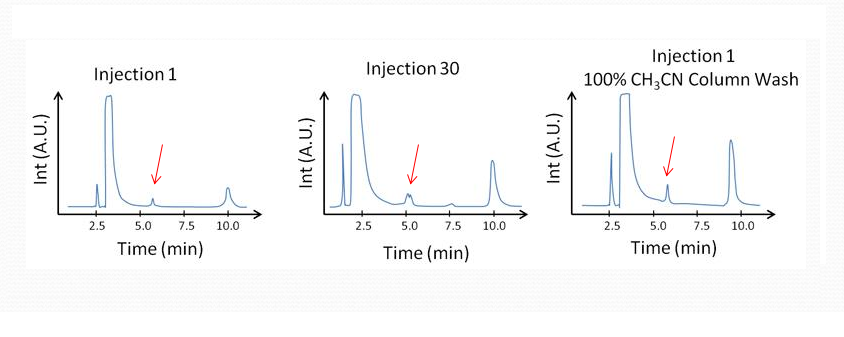
Split Peaks from Column Contamination
•Column: StableBond SB-C8, 4.6 x 150 mm, 5 μm Mobile Phase: 60% 25 mM Na2HPO4, pH
3.0 : 40% MeOH Flow Rate: 1.0 mL/min
Temperature: 35°C Detection: UV 254 nm Sample: Filtered OTC Cold Medication: 1.
Pseudoephedrine 2. APAP 3. Chlorpheniramine
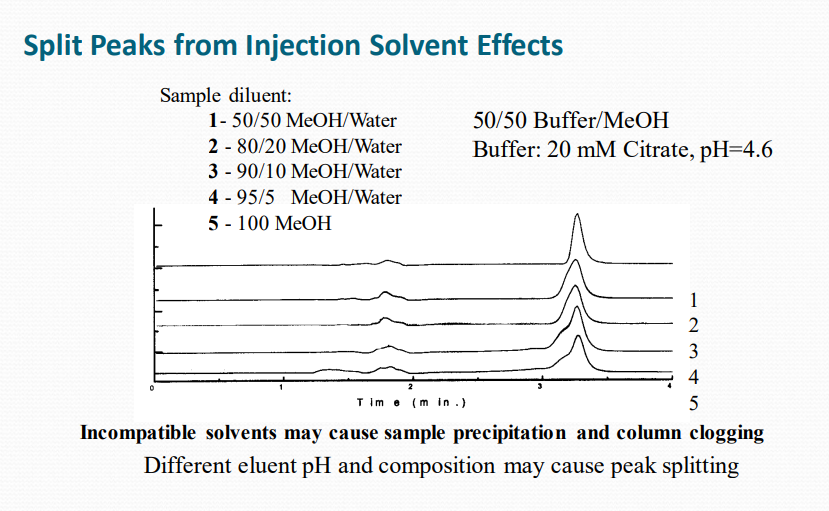
Peak Tailing, Broadeningand Loss of Efficiency
May be caused by:
➢Column “secondary interactions”
➢Column contamination
➢Column aging
➢Column loading
➢Extra-column effects
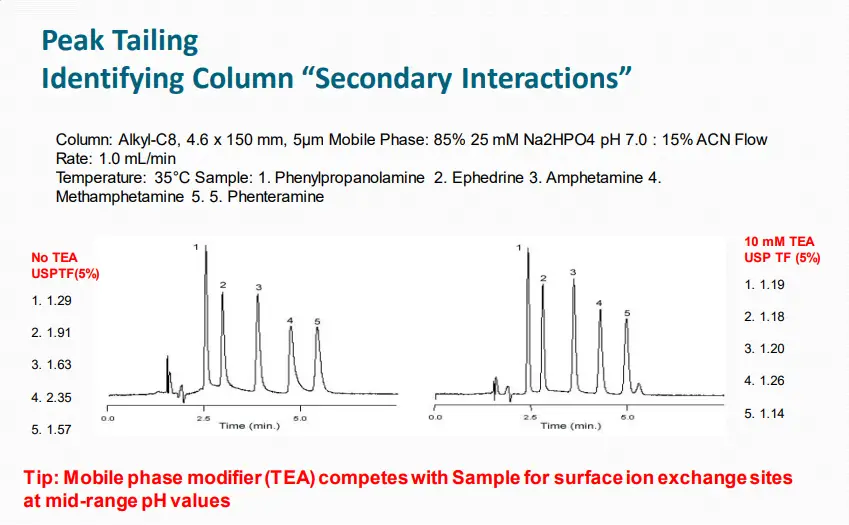
Low pH Minimizes “Secondary Interactions” for Amines
Column: Alkyl-C8, 4.6 x 150 mm, 5μm Mobile Phase: 85% 25 mM Na2HPO4 pH 7.0 : 15% ACN Flow
Rate: 1.0 mL/min
Temperature: 35°C Sample:
1. Phenylpropanolamine
2. Ephedrine
3. Amphetamine
4. Methamphetamine.
5. Phenteramine
Tip: Reducing mobile phase pH reduces interactions with silanols and peak tailing.
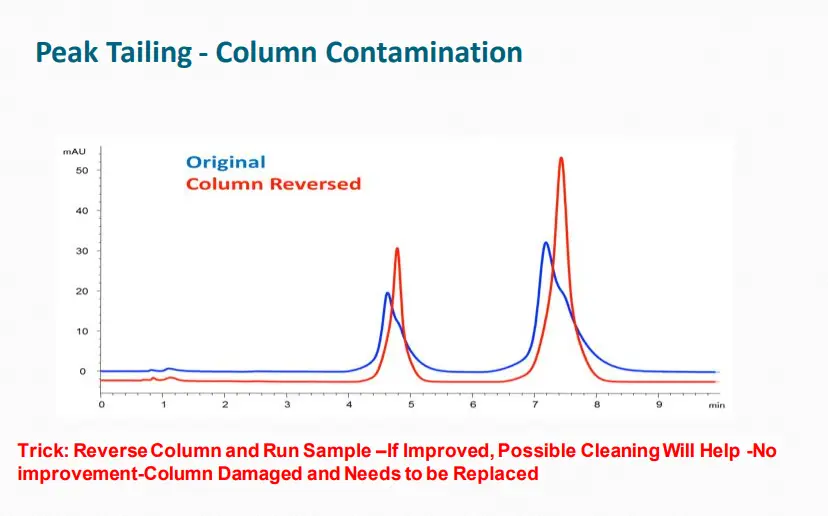
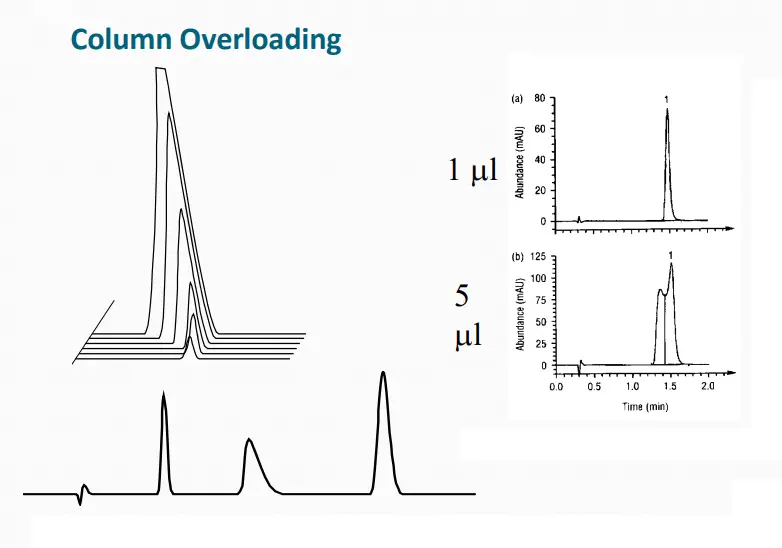
Peak Shape: Broad Peaks
All Peaks Broadened:
➢ Loss of Column Efficiency.
➢ Column Void.
➢ Large Injection Volume
Some Peaks Broadened:
➢ Late Elution from Previous Sample (Ghost Peak).
➢ High Molecular Weight.
➢ Sample – Protein or Polymer.
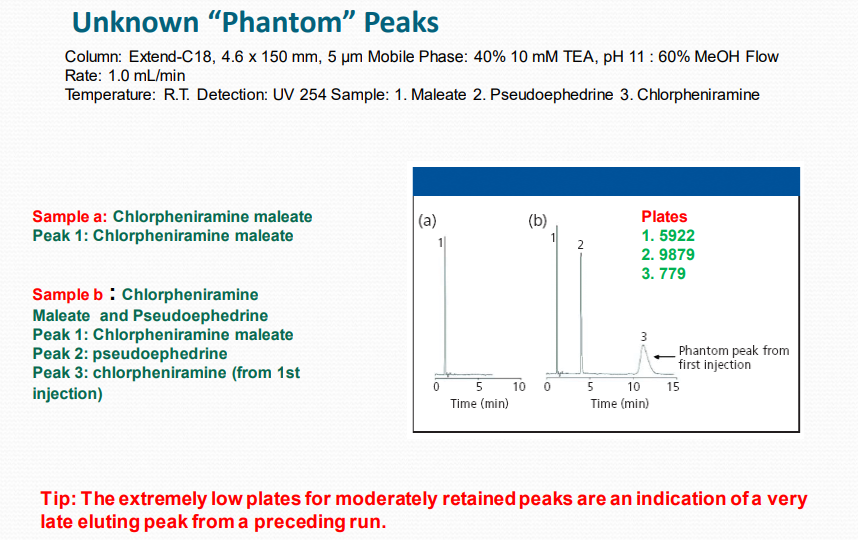
III. Changes in Retention Time
Changes in Retention Can Be Chemical or physical and May be caused by:
➢Column aging
➢Column contamination
➢Insufficient equilibration
➢Poor column/mobile phase combination
➢Change in the mobile phase
➢Change in flow rate
III. Changes in Retention Time
Mobile Phase pH and pH Buffers Why Are These So Important in HPLC?
pH Effects Ionization
➢Silica Surface of Column
➢Sample Components of Interest Buffers
➢Resist Changes in pH and Maintain Retention
➢Improve Peak Shape for Ionizable Compounds
Effects Column Life
➢Low pH strips Bonded Phase
➢High pH Dissolves Silica
Dependencies of Analyte Retention on the pH of the Mobile Phase
Ionization in general decreases hydrophobicity causing a decrease of HPLC retention.
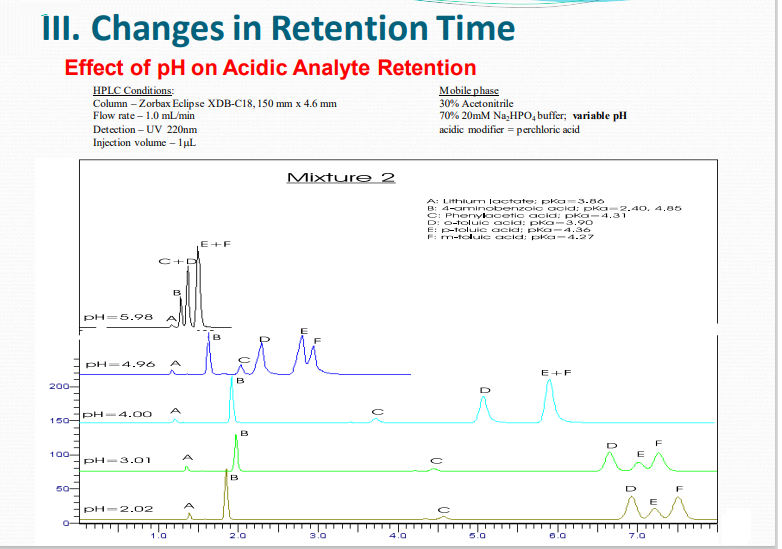
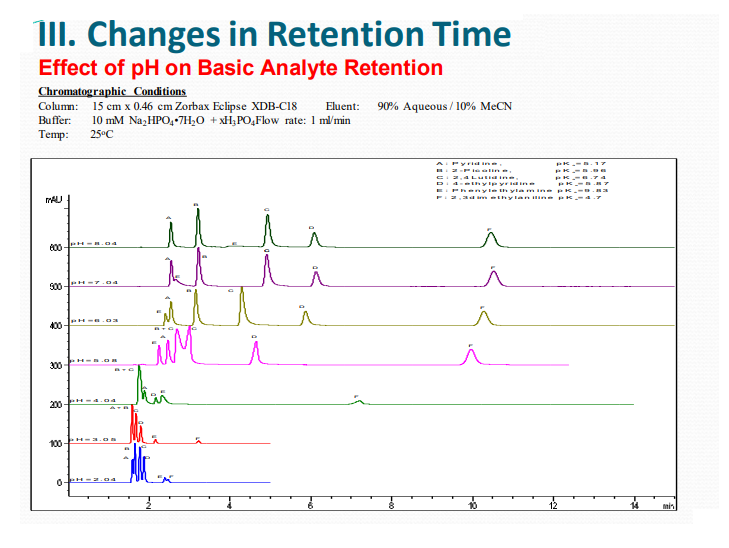
Importance of pH and Buffers
➢ pH is an effective tool for adjustment of selectivity and retention
➢ pH can be used to optimize the resolution
➢ Reversed phase packaging is most stable between pH’s 2 – 8.
➢ Don’t Forget – Match Column to pH of mobile phase for maximum column lifetime
IV. Detection Issues
Recognize Where the Problem Originates
➢ Is it a consequence of technique?
➢ Is It expected due to the use of certain mobile phase components?
➢ Can it be corrected by adjusting detector parameters?
Drifting Baselines
➢ Detector (UV) not set at absorbance maximum but at the slope of the curve
➢ Gradient Elution
➢ Temperature Unstable (Refractive Index Detector)
➢ Contamination in Mobile Phase
➢ Mobile Phase Not in Equilibrium with Column
Baseline Noise
➢ Mobile phase contaminated, deteriorated, or prepared from low-quality materials
➢ Mobile phase solvents are immiscible
➢ Air trapped in the system
➢ Air bubbles in a detector
➢ Detector cell contaminated (even small amounts of contaminants can cause noise)
➢ Weak detector lamp
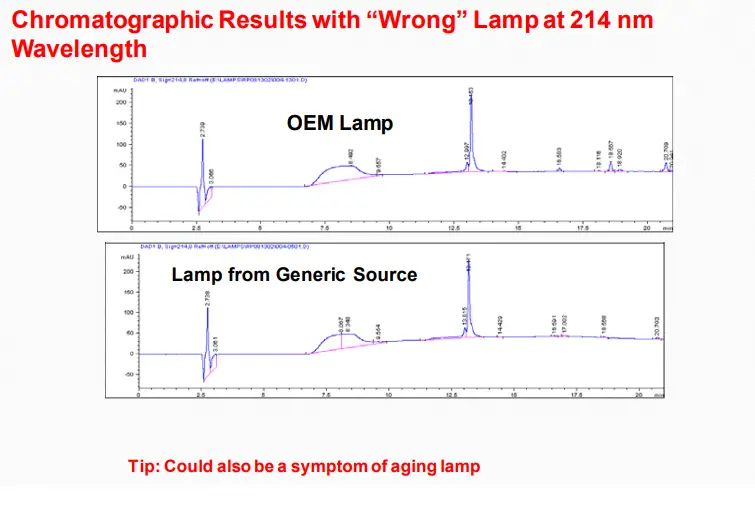
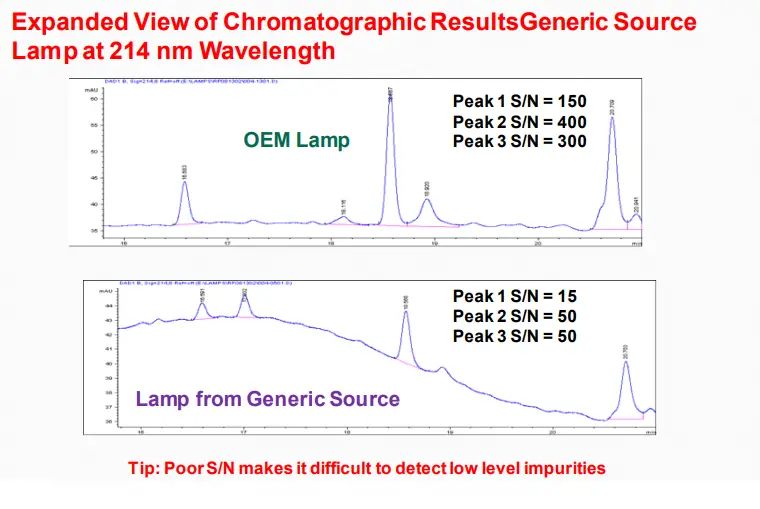
Conclusions
➢HPLC column problems are evident as
▪ High pressure (prevention better than the cure)
▪ Undesirable peak shape
▪ Changes in retention/selectivity
Often these problems are not associated with the column and may be caused by instrument and chemistry issues.
➢ pH of mobile Phase
➢ Instrument Connections
➢ Detector Settings
➢ Metal Contamination
Start With the Correct Questions
➢ Find the Answers &The Answers will Lead to Solutions
