DOCUMENTATION IN PHARMA Well-established documentation system exists to implement Good Documentation Practices, which is a part of Quality Assurance. Clearly written documents are prepared, reviewed and controlled, which prevents the errors from verbal communication and permits traceability for all activities carried out. Quality Assurance is responsible for the revision and …
Read More »WATER SYSTEM
WATER SYSTEM: A purified Water system is a basic requirement for Pharmaceutical Industries. Raw Water sourced from industry bore well water is converted into purified water complying with current USP/ BP/ IP/ Ph. Eur. GENERATION OF PURIFIED WATER: The raw water passes through a sand filter through a transfer pump. …
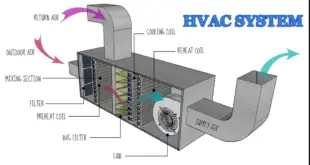
Read More »HEATING, VENTILATION AND AIR CONDITIONING (HVAC) SYSTEMS
BRIEF DESCRIPTION OF HEATING, VENTILATION, AND AIR CONDITIONING (HVAC) SYSTEMS: To avoid cross-contamination and to maintain relative humidity, temperature, and Pressure Difference of the manufacturing areas with Air handling Units, Air handling Units Provided in the manufacturing, warehouse, quality control, and microbiology section. Temperature and humidity are controlled and maintained …
Read More »BATCH MANUFACTURING RECORD OF DRY INJECTION
BATCH MANUFACTURING RECORD OF DRY INJECTION Table of content Batch Details General Instructions Calculation of API Dispensing of raw material API transfer record Dispensing of primary packing materials Component preparation for sterilization Steam sterilization record Vial de-boxing, Staging, and Inspection record Vial washing record Vial depyrogenation record API Canister weight …
Read More »WHO TRS1019 Appendix 3 – Cleaning validation Principle
WHO TRS1019 Appendix 3 – Cleaning validation Principle Content of Cleaning validation Principle 1.0 Principle 2.0 Scope 3.0 General 4.0 Cleaning validation protocols and reports 4.1 Cleaning validation protocols 4.2 Cleaning validation reports 8.0 Personnel 6.0 Equipment 7.0 Detergents 8.0 Microbiology 9.0 Sampling 9.1 General 9.2 Direct surface sampling (direct …
Read More »PERSONNEL HYGIENE
PURPOSE OF PERSONAL HYGIENE: To lay down the procedure for to ensure for Personnel hygiene of all employees working in the company. SCOPE OF PERSONNEL HYGIENE: This SOP is applicable for all employees working at Pharmaceuticals Company. RESPONSIBILITY OF PERSONNEL HYGIENE Preparation of SOP: HR Executive /Officer Checking and Review …
Read More »Quality Risk Management in Analytical Laboratory
Quality Risk Management in Analytical Laboratory The Quality of the Product Drugs or Drug Product is evaluated in Quality Control analytical laboratories, So It is important to understand that the “services” and “output” of a Quality Control / Analytical Laboratory is directly related to Safety, Identity, Strength, Purity (efficacy), Quality …
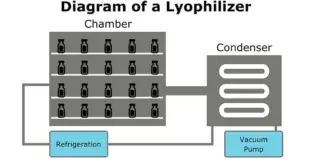
Read More »Lyophilization of Parenteral
Lyophilization of Parenteral Lyophilization or freeze-drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase. The process consists of three separate, unique, and …
Read More »DRUG DESIGN (AN OVERVIEW)
DRUG DESIGN (AN OVERVIEW) DRUG DESIGN DRUG DESIGN Approaches to drug discovery: Serendipity (luck) Chemical Modification Screening Rational Irrational, based on serendipity & Intuition Trial & error approach in DRUG DESIGN Time consuming with low through output No de novo design, mostly “Me Too Approach” First generation Rational approach in …
Read More »CHANGE CONTROL
CHANGE CONTROL Change Control is “A formal system by which qualified representatives of appropriate disciplines review proposed or actual changes that might affect the validated status of facilities, systems, equipment or processes. The intent is to determine the need for action that would ensure and document that the system is …
Read More »Quality Management System
Quality Management System Definition: A Quality Management System is a collection of policies, procedures, plans, resources, processes, practices, and the specification of responsibilities and authority of an organization designed to achieve product and service quality levels, customer satisfaction, and company objectives. Documentation: Quality Policy – describes the organization’s approach to …
Read More »HAZARD ANALYSIS OF CRITICAL CONTROL POINT (HACCP)
HAZARD ANALYSIS OF CRITICAL CONTROL POINT (HACCP) TABLE OF CONTENTS HAZARD ANALYSIS OF CRITICAL CONTROL POINT 1. Declaration. Quality Policy Quality Objectives Company Profile Layout of Plant Organization Chart HACCP Introduction HACCP Prerequisite Programs Seven Principles of HACCP Hazards HACCP Scope HACCP Team Responsibility Hazard Analysis Introduction Hazard Analysis for …
Read More »