Current Trends in Cleaning Validation
- Rings on Buffer and Media Tanks
- Health based limits
- FDA Trends
- Process Understanding
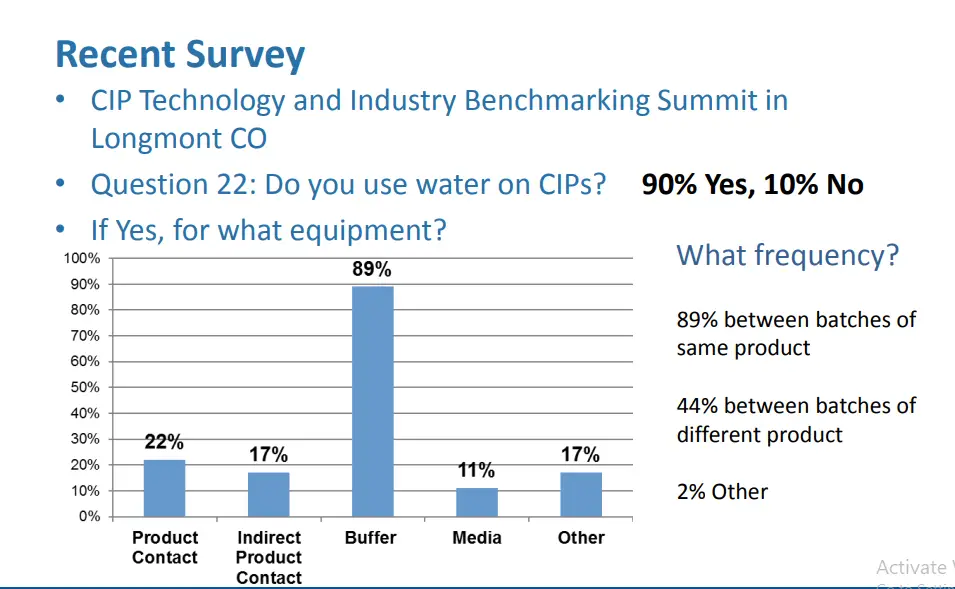
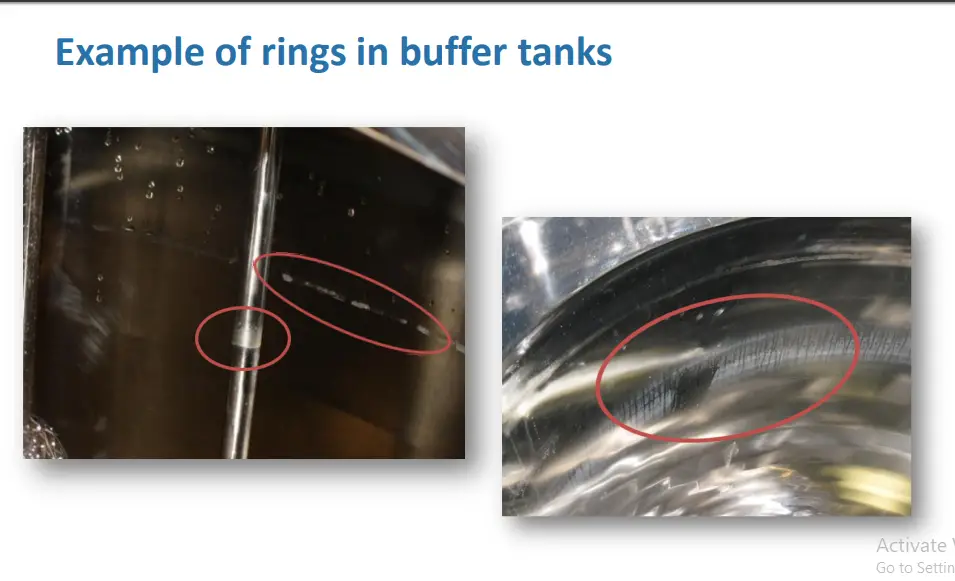
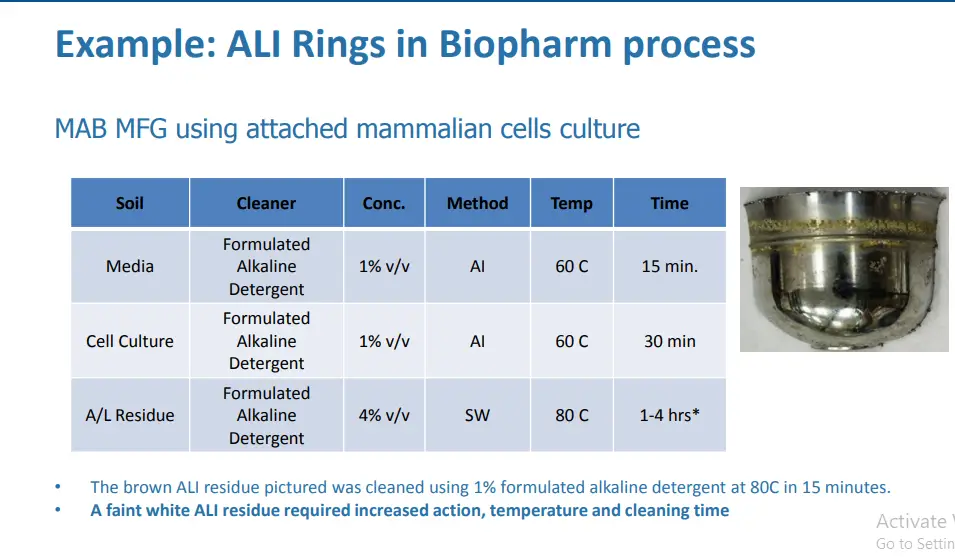
Trend 1: Rings in Buffer tanks
Initial Incident Discovery
Background
• Cleaning processes are designed based on
– nature of the soil
– condition of the soil
– surface material
– cleaning factors
• ALI rings can be challenging to clean
• This is not a new issue however the subject has received a lot of attention recently
Air-Liquid Interface Residues
• Trace elements are normally at ppb levels
• Normally hydrophobic and migrate to surface
• High mixing speeds assist with migration to surface and adherence to side walls
• Large liquid volumes increase trace residues present
• Small surface area at ALI allows concentration of residues at ALI
• Hot water rinses can heat surface and bake residues onto side walls
Air – Liquid Interface Residues
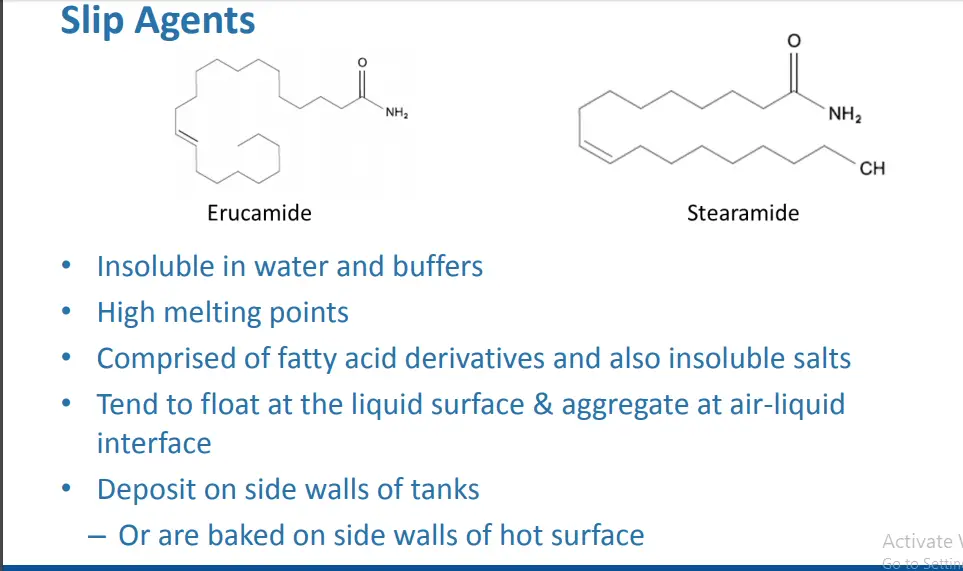
Raw material impurities
– Hydrocarbons (Steramide, Erucamide, Oleamide)
• Mold release agents used in processing of bags & and equipment
• Prevents caking of powders
• Found on bags used for storage and transport
– Polymers (Teflon, Nylon, PTFE, Silicone, etc.)
• Equipment used in raw material manufacturing
• Bags or containers used for raw material storage
• Impurities from source material
– Mineral Silicates (anti-blocks) (Silica, Talc, Kaolin)
– Lubricants
– Valves, gaskets and tubing – (source for siloxanes)
Industry Awareness
• Raw materials used in buffer solutions are stored and transported in bins lined with plastic bags or in the raw
materials themselves.
• Plastic bags (poly liners) are manufactured from films made by extrusion for a range of purposes and industries.
• Slip and anti-block additives are added to resins to promote slip between the layers of the film to reduce friction and ‘tackiness’ to increase the ability of the material to slide over itself
• Unintended consequence: trace amounts mix with the materials stored in the plastics liners and subsequently appear in bioprocess preparation tanks.
Filtration of slip agents
• Filtration studies show that slip agents do not pass through filters
– Exception could be for detergent based buffers for example S/D buffers
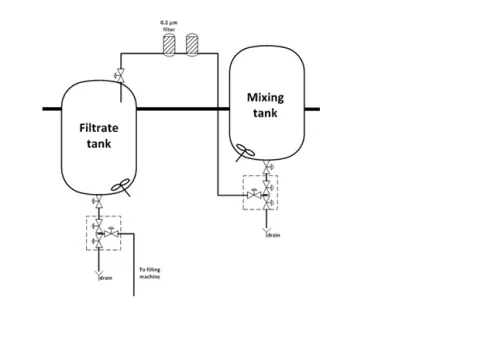
– Recently seen at Customer site where compounding tank has issue with rings and filtrate tank does not
Industry Awareness
Set specification in user requirement definitions for suppliers to remove from material/processes
– Benchmarking showed that specifying ‘no slip agents’ was not customary.
• Research showed that low-density polyethylene and linear low-density polyethylene liners are the most frequently used liners in packaging raw materials. Many grades include the use of slip and anti-block agents but some grades do not.
What the raw material vendors do
• How do bag and slip agent manufacturers clean their own equipment?
– Solvents
• Involve manual wipe down
• Flammable
• Not suited for CIP of pharma & biopharma equipment
• Key is to dissolve hydrophobic residues in aqueous solutions
Common Cleaning Approaches
• Increase spray impingement by using rotating spray device
• Increase temperature of cleaning (75-85C)
• Use of formulated cleaning agent
• Increase cleaning agent concentration
• Use of an oxidizing cleaning agent or detergent additive with an alkaline cleaning solution
– Keep temp > 50°C to < 65°C
• Formulated alkaline detergent alone or in combination with a formulated oxidative detergent or surfactant based
detergents.
Example of recommended cleaning frequency
• Group 1: Very High: Halide Salt Buffers > 5M: Clean & Passivate after every formulation
• Group 2: High: Halide Salt Buffers 2 – 5M: Clean & Passivate after every 10 formulations
• Group 3: Moderate: Halide Salt Buffers 1 to 1.5M: Clean & Passivate after every 20 formulations
• Group 4: Low: Halide Salt Buffers 0.1 to 1M: Clean & Passivate after every 50 formulations
• Group 5: Very Low: Halide Salt Buffers <0.1M: Clean & Passivate after every 100 formulations
Conclusions
• Identify components of the ring
• Determine whether intrinsic or extrinsic to process
– Intrinsic: modify cleaning procedure
– Extrinsic: try to eliminate source or modify cleaning procedure
• PACE evaluation can be a tool to assist in the
investigation or corrective action
• Risk assessment should be performed
• Visible residues may need to be manually cleaned
• Routine cleaning with a formulated cleaning agent containing surfactants at elevated temperature may prevent visible residue buildup and avoid manual cleaning.
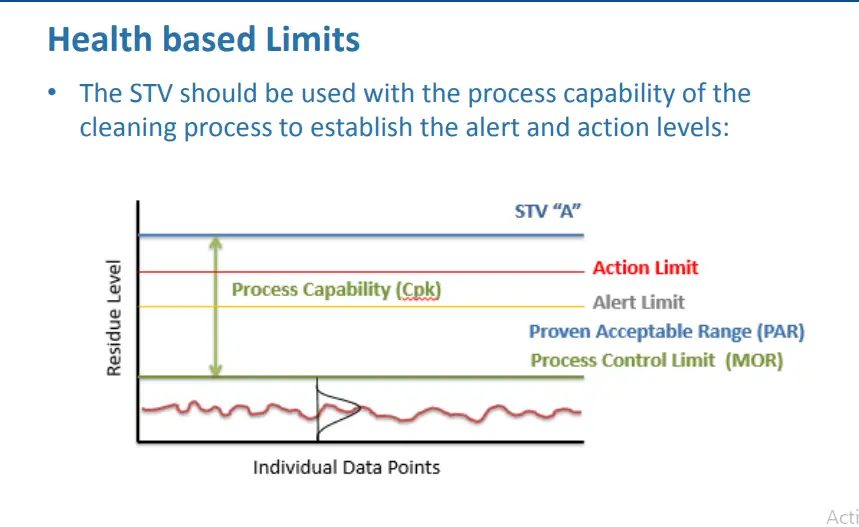
Trend 2: Health Based Limits
What is cleaning for?
• Cleaning has to do with what is manufactured next
• Need information for subsequent product:
– Formulation
– Dosing
– Route of administration
– Batch size
– Shared equipment
– Product specifications (for bioburden and LAL specs)
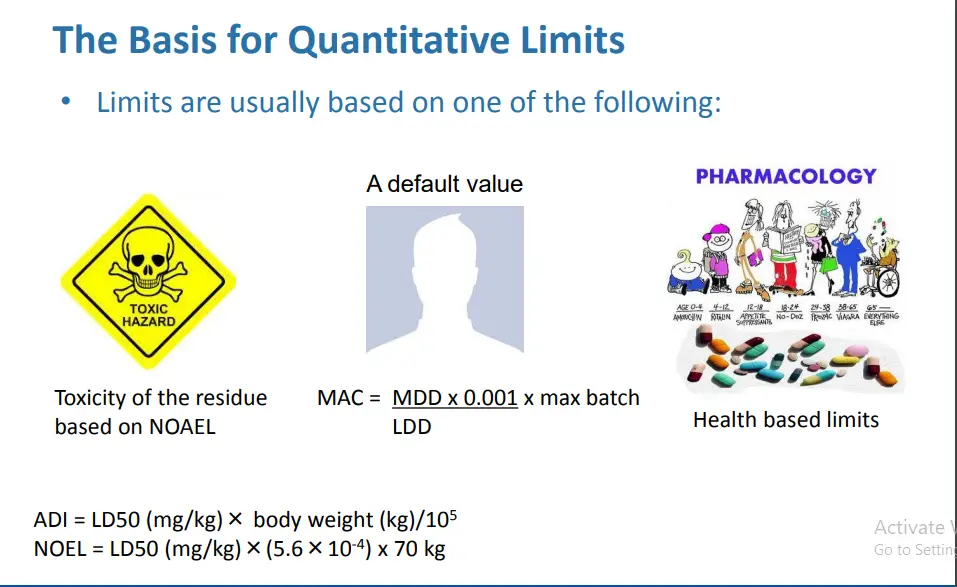
Health Based Limit
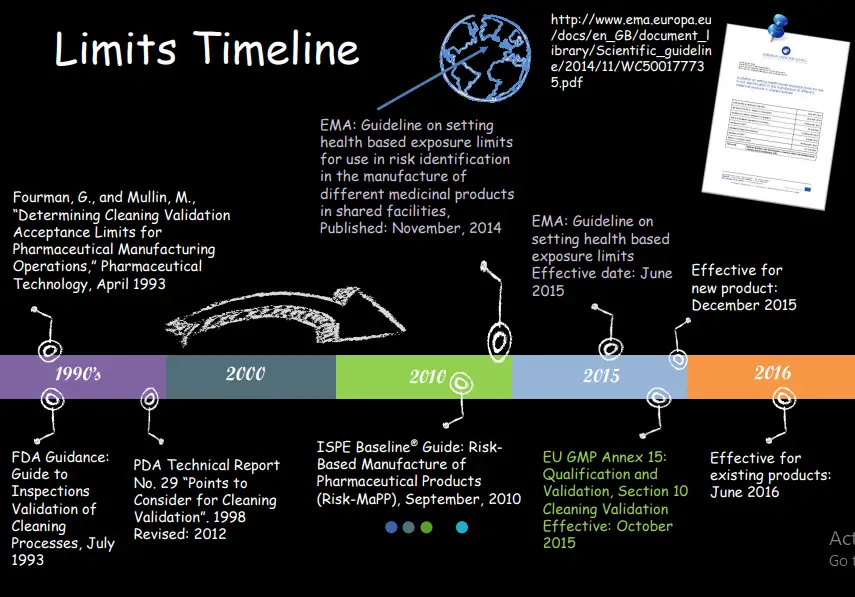
• ISPE Risk MaPP and recent EMA document are two main advocates & EU GMP Annex 15
– ADE: Acceptable daily exposure
– PDE: Permitted daily exposure
• Refers to limits based on toxicological evaluation
– Focuses on how carryover might cause harm.
– Value represents dose that is unlikely to cause an adverse effect if an individual is exposed at this dose every day for a lifetime
• Why?
– Some felt that “traditional” method was not “risk-based”
– Use of 1/1000th daily dose of active seen by some to be “arbitrary”
– Some questioned the use of 10 ppm as the “default” value as arbitrary and possibly not reflective of potential risks
– Current approach could lead to either overly prescriptive limits or inadequate limits
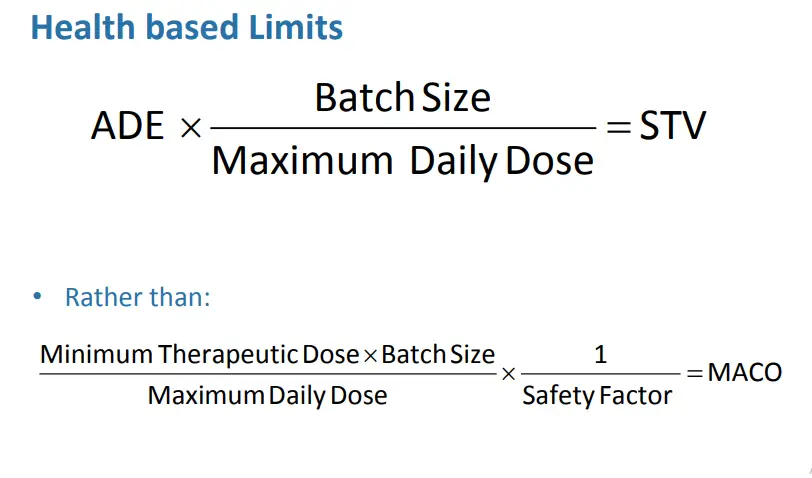
Calculation of ADE Value
• Per ISPE Baseline®Guide: Risk-Based MaPP
– ADE should not be seen as a “limit”
• Initial acceptance criteria are limits safe for the patient
– Use as a reference point for determining level of risk
– Establish Process Control Limits based on PD Studies
• Tighter inner control limits (MOR’s & PAR’s)
• Calculated per statistical analysis of CV data and monitoring data
• ADE limit alone may not be acceptable as carryover,though considered safe
– Flavor, smell, product quality, etc.
– Default to visually clean
• Several critical effects identified resulting in the calculation of more than one PDE value:
– Use lowest PDE value
• Two (or more) different values from different Toxicologists:
– Use lowest PDE value
• Rationale for having some check on an ADE/PDE only approach
– NOTE: FDA has questioned this with respect to process control capabilities.
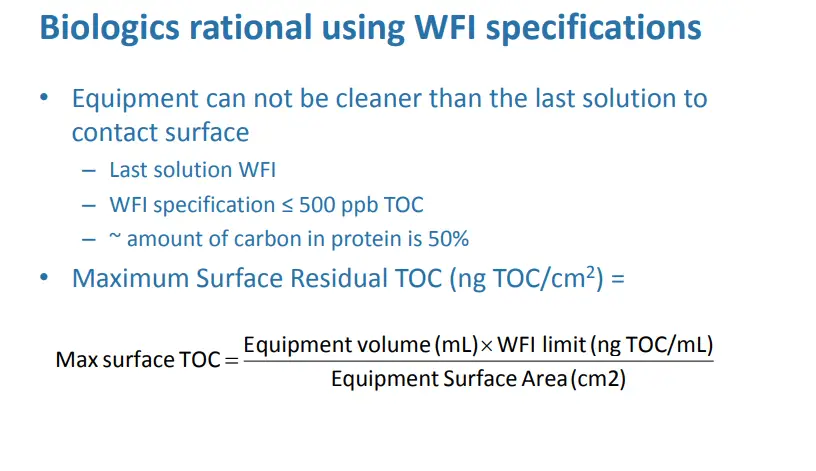
Issues with Biologics due to denaturation
• EMA “Guideline on setting health based limits…” 20 Nov
14
– Section 5.3 “In view of this, the determination of health based exposure limits using PDE limits of the active and intact product may not be required.
• EudraLex, Volume 4, “EU Guidelines for GMP for Medicinal Products…Annex 15: 30 Mar 15
– Section 10.6.1: “Therapeutic macromolecules…known to degrade and denature when exposed to pH extremes and/or heat…A toxicological evaluation may therefore not be applicable…”
Issues with Biologics due to denaturation
• Active is degraded after cleaning
– Irrational to use product specific assays
• Acceptance limits often below LOD of non-specific methods
– Large surface areas/small batches/low doses
Limits based on thresholds
• Regulatory Toxicology and Pharmacology 43 (2005) 1-9,Dolan, D., Naumann, B., Application of the threshold of
toxicological concern concept to pharmaceutical manufacturing operations.
– ADI recommendations based on threshold values for resolution of atypical extraneous matter investigations
• Carcinogens: 1µg/day
• Unstudied compounds with limited data: 10 µg/day
• Compounds not likely potent, highly toxic or carcinogenic: 100 µg/day
FDA Trends
FDA Strategic Priorities
• FDA has indicated that the following areas are (GMP)
inspectional priorities: (risk based inspection program)
– High risk products
– Prescription drug manufacturers
– Sterile drug manufacturers
– New facilities not yet inspected
• Important priorities
– Assessment of “Breakthrough Therapies” program
– Expedited review programs
Why a sound cleaning program is important?
• Cleaning shows an Auditor a lot about a facility
– Quality mindset – How site makes decisions under manufacturing schedule stress (first patient in)
– How they investigate deviations
– Do they understand the process?
Process Understanding
Do you understand your process?
• Meet with your team or “Customer”
– Discuss process residue details
• Aqueous, oil based, suspension, solids, powders, etc
– Equipment surface MOC
– Available cleaning methodology options
– Process temperature and process details
– Decontamination step?
– Dirty hold time
– Restrictions?
• Schedules, waste, limitations
In Conclusion….
• Building an effective cleaning program involves
– Understanding your process
• Equipment design considerations
• Process considerations
– Evaluation of soiling conditions
– Establishing correct limits, both cleaning validation and incoming raw material specifications (or testing worse case for process capability)
Referances: PPT on Current Trends in Cleaning Validation Beth Kroeger, STERIS Technical Services Manager ,Beth_Kroeger@steris.com