Tablets In Pharma Industry
NOTE- The provisions of this monograph do not necessarily apply to tablets intended for use other than by oral
administration such as Vaginal preparations or Or mucosal preparations, and to lozenges, oral pastes and oral gums.
Introduction
Tablets are solid dosage forms each containing a unit dose of one or more medicaments.
They are intended for oral administration. Some tablets are swallowed whole or after being chewed, some ate dissolved or dispersed in water before administration and some are retained in the mouth where the active ingredient is liberated.
Because of their composition, method of manufacture or intended use, tablets present a variety of characteristics and
consequently there are several categories of tablets.
Tablets may be coated. Where coating is essential, the monograph states ‘The tablets are coated’.
In all other cases, coating is optional. Unless otherwise directed, tablets may be coated in one of different ways.
Tablets are usually solid, right circular cylinders, the end surfaces of which are flat or convex and the edges of which
may be bevelled. They may exist in other shapes like triangular, rectangular, etc also.
They may have lines or break-marks and may bear a symbol or other markings.
They are sufficiently hard to withstand handling without crumbling or breaking.
Production In Pharma Industry
Tablets are obtained by compression of uniform volumes of powders or granules by applying high pressures and using punches and dies.
The particles to be compressed consist of one or more medicaments, with or without auxiliary substances such as diluents, binders, disintegrating agents, lubricants, glidants, permitted colours and substances capable of modifying the behaviour of the medicaments in the digestive tract.
Such substances must be innocuous and therapeutically inert in the quantities present.
In the production of tablets, measures are taken to ensure that they have sufficient strength to avoid crumbling or breaking on handling or subsequent handling.
Chewing tablets are manufactured to ensure that they are easily crushed by chewing.
During manufacture, packaging, storage and distribution of tablets, suitable means shall be taken to ensure their microbial quality; acceptance criteria for microbial quality are given in Chapter 5.9.
Tests In Pharma Industry
NOTE – Unless otherwise stated below or in the individual monograph, the following tests apply to all categories of
tablets.
Uniformity of container contents. Tablets comply with the test for contents of packaged dosage forms (2.5.6).
Content of active ingredients. Determine the amount of active ingredient(s) by the method described in the Assay and calculate the amount of active ingredient(s) per tablet.
The result lies within the range for the content of active ingredient(s) stated in the monograph.
This range is based on the requirement that 20 tablets, or such other number as may be indicated in the monograph, are used in the Assay.
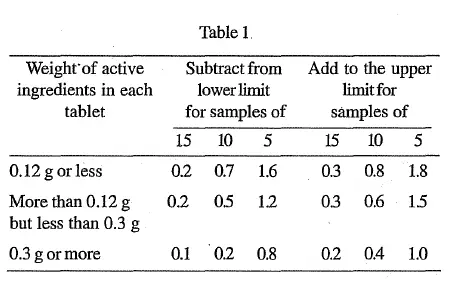
Where 20 tablets cannot be obtained, a smaller number, which must not be less than 5, may be used, but to allow for sampling errors the tolerances are widened in accordance with Table 1.
The requirements of Table 1 apply when the stated limits are between 90 and 110 per cent. For limits other than 90 to 110 per cent, proportionately smaller or larger allowances should be made.
Uniformity of content (2.5.4).
This test is applicable to tablets that contain 10 mg or less than 10 mg or less than 10 per cent w/w of active ingredient. For tablets containing more than one active ingredient carry out the test for each active ingredient that corresponds to the aforementioned conditions.
The test is also applicable to coated tablets other than film-coated tablets, irrespective of their content of active
substance(s).
The test for Uniformity of content should be carried out only after the content of active ingredient(s) in a pooled sample of the tablets has been shown to be within accepted limits of the stated content.
The test for Unifonnity of content is not applicable to tablets containing multivitamins and trace elements.
Uniformity of weight (2.5.3).
This test is not applicable to coated tablets other than film-coated tablets and to tablets
that are required to comply with the test for uniformity of content for all active ingredients.
Dissolution (2.5.2).
Where required, the requirements for this test are given in the individual monographs. Where a dissolution test is prescribed, the disintegration test may not be necessary.
Uncoated Tablets
Uncoated tablets may be single-layer tablets resulting from a single compression of particles or multi-layer tablets consisting of parallel layers obtained by successive compression of particles of different compositions.
No treatment is applied to such tablets after compression.
Any added substances are not specifically intended to modify the release of their active ingredient(s) in the digestive fluids.
The addition of flavouring agents to uncoated tablets other than multi-layer tablets is not official unless permitted in the individual monograph. Uncoated tablets have the general characteristics of tablets.
When a broken section of an uncoated tablet is examined under a lens, either a relatively uniform texture (single-layer tablets) or a stratified structure (multi-layer tablets) is seen; there are no signs of coating.
Tests
Disintegration (2.5.1).
Use water as the liquid. Add a disc to each tube. Operate the apparatus for 15 minutes, unless otherwise stated in the individual monograph. Examine the state of the tablets. If the tablets fail to comply because of adherence to the discs, repeat the test on a further 6 tablets omitting the discs, The tablets comply with the test if all 6 tablets have disintegrated. The test does not apply to chewable tablets.
Coated Tablets
Coated tablets are tablets covered with one or more layers of mixtures of various substances such as resins, gums, inactive and insoluble flliers, sugars, plasticisers, polyhydric alcohols, waxes, etc.
The coating may also contain medicaments. In compression coated-tablets, the coating is applied by compressing around the tablets granules prepared from tablet excipients such as lactose, calcium phosphate, etc.
Substances used as coatings are usually applied as a solution or suspension in conditions in which evaporation of the vehicle occurs. When the coating is thin, the tablets are described as fllm-coated.
Coated tablets may contain flavouring agents.
Coated tablets have a smooth, usually polished and often coloured, surface; a broken section examined under a lens
shows a core surrounded by one or more continuous layers of a different texture.
Tests
Disintegration (2.5.1).
For coated tablets other than film-coated tablets.
Use water as the liquid. Add a disc to each tube. Operate the apparatus for 60 minutes, unless otherwise stated in the
individual monograph. Examine the state of the tablets, If any of the tablets has not disintegrated, repeat the test on a further 6 tablets, replacing water with O.1.M hydrochloric acid.
The tablets. comply with the test if all 6 tablets have disintegrated in the acid medium.
Film-coated Tablets
Carry out the test described above but operate the apparatus for 30 minutes, unless otherwise stated in the individual Monograph.
If coated tablets fail to comply because of adherence to the discs, repeat the test on a further 6 tablets omitting the discs.
The tablets comply with the test if all 6 tablets have disintegrated.
The test does not apply to chewable tablets.
Dispersible Tablets
Dispersible tablets are uncoated or film-coated tablets that produce a uniform dispersion in water and may contain
permitted flavouring and sweetening agents.
However, if saccharin, including its sodium and potassium salts, is used as a sweetening agent, its concentration in dispersible tablets meant for paediatric use should be restricted so as to limit its intake to 5 mg kg of body weight.
Tests
Disintegration (2.5.1).
Determine at 24″ to 26″ and operate the apparatus for 3 minutes.
Uniformity of dispersion.
Place 2 tablets in 100 ml of water and stir gently until completely dispersed. A smooth dispersion is obtained which passes through a sieve screen with a nominal mesh aperture of 710 μm (sieve number 22).
Effervescent Tablets
Effervescent tablets are uncoated tablets generally containing acidic substances and either carbonates or bicarbonates which react rapidly in the presence of water to release carbon dioxide.
They are intended to be dissolved or dispersed in water before administration.
Tests
Disintegration (2.5.1).
Place one tablet in a 250-ml beaker containing water at 20º to 30º; numerous gas bubbles are evolved. When the evolution of gas around the tablet or its fragments has ceased the tablet shall have disintegrated, being either dissolved or dispersed in the water so that no agglomerates of particles remain.
Repeat the operation on a further 5 tablets. The tablets comply with the test if each of the 6 tablets disintegrates in the manner prescribed within 5 minutes unless otherwise stated in the individual monograph.
Modified-release Tablets
Modified-release tablets (Sustained-release tablets) are coated or uncoated tablets containing auxiliary substances or
prepared by procedures that, separately or together, are designed to modify the rate or the place at which the active
ingredient is released.
Modified-release tablets include enteric-coated tablets, prolonged-release tablets and delayed-release tablets.
Enteric-coated Tablets
Enteric-coated tablets (Gastro-resistant tablets) are delayed-release tablets that are intended to resist the gastric fluid but to release their active ingredient(s) in the intestinal fluid.
For this purpose substances such as cellulose acetate phthalate and anionic copolymers of methacrylic acid and its ethers are used for providing tablets with a gastric-resistant coating or for covering either granules or particles with gastric-resistant coating.
Enteric-coated tablets have the characteristics of Coated Tablets.
Tests
Disintegration (2.5.1).
If the tablet has a soluble external coating, immerse the basket in water at room temperature for 5 minutes. Suspend the assembly in the beaker containing 0.1 M hydrochloric acid and operate without the discs for 120 minutes, unless otherwise stated in the individual monograph. Remove the assembly from the liquid. No tablet shows signs of cracks that would allow the escape of the contents of disintegration, apart from fragments of coating.
Replace the liquid in the beaker with mixed phosphate buffer pH 6.8, add a disc to each tube and operate the apparatus for a further 60 minutes. Remove the assembly from the liquid. The tablets pass the test if all six have disintegrated.
Dissolution (2.5.2).
For tablets prepared from granules or particles already covered with an enteric coating, the dissolution test is carried out to demonstrate the appropriate release of the active substance(s).
Prolonged-release Tablets
Prolonged-release tablets, also known as sustained-release tablets or extended-release tablets are tablets formulated in such a manner as to make the contained active ingredient available over an extended period of time after ingestion.
Tests
Dissolution (2.5.2).
The test should be designed to demonstrate the appropriate release of the active substance(s).
The manufacturer is expected to give specifications for drug release at 3 or more test-time points. The first point should be set after a testing period corresponding to a dissolved amount of typically 20 per cent to 30 per cent.
The second point should define the dissolution pattern and should be set at around 50 percent release. The final point should ensure almost complete release that is generally understood as more than 80 percent release.
Carry out the test as per the manufacturer’s specification for the indicated test-times.
Soluble Tablets
Soluble tablets are uncoated tablets or film-coated tablets that are to be dissolved in water before use. The solution produced may be slightly opalescent due to added substances used in the manufacture of the tablets.
Tests
Disintegration (2.5.1).
Soluble tablets disintegrate within 3 minutes. The test is carried out using water at 15° to 25°.
Tablets for Use in the Mouth
Tablets for use in the mouth are usually uncoated tablets formulated to be chewed or to effect a slow-release and local action of the active ingredient (lozenges) or the release and absorption of the active ingredient under the tongue
(sublingual tablets). Chewable .tablets and lozenges may contain flavoring agents.
Labelling.
The label states whether or not the tablets are coated.
Where applicable the label states that the tablets should be chewed before swallowing.