SOP FOR STABILITY STUDY IN PHARMA INDUSTRY
OBJECTIVE:
To lay down the procedure for collection, storage and analysis of stability of samples.
SCOPE:
This SOP shall be applicable for stability study of samples in Quality Control Department in Pharma Industry.
RESPONSIBILITY:
Officers/Executive-Quality Control shall be responsible for follow that procedure.
ACCOUNTABILITY:
Manager- Quality Control and Head QA shall be accountable for compliance of this SOP.
ATTACHMENT:
| Product List in the stability chamber | – | Attachment-I |
| Stability Studies Calendar | – | Attachment-II |
| Monthly Record of Stability Samples Withdrawal | – | Attachment-III |
| Stability Samples Register | – | Attachment-IV |
| Stability Study Protocol | – | Attachment-V |
| Stability Study Report | Attachment-VI | |
| Stability Samples Maintenance Record | – | Attachment-VII |
PROCEDURE:
Stability Program
The purpose of the stability studies is to evaluate the product stability during its shelf period. The stability studies are conducted at three conditions:
- Accelerated Stability.
- Intermediate Stability.
- Long Term Stability.
Accelerated stability studies
Storage conditions: Storage temperature is 40 ± 2°C and RH is 75 ± 5%.
The Stability Study samples should be kept in the marketable pack or equivalent.
Testing intervals: 0M, 3M and 6M.
Long-term stability studies.
Storage conditions: temperature is 25 ± 2°C and RH is 60 ± 5%.
The stability study samples should be kept in the marketable pack or equivalent.
Study duration: shelf life period + 1year.
Testing intervals:
- First year: once in three month.
- Second year: once in six month.
- After second year: once in a year.
The selection criteria of batches to be kept on stability studies should be as follows:
- First 3 validation batches of the new product;
- One commercial batch every year after 3 consecutive validation batches;
- First 3 batches of the product after any change taking place in API and (or) excipient, manufacturing process, primary packaging materials;
- Batches after reprocessing or reworking;
- Placebo coinciding with each batch placed on stability tests.
Sampling:
Prepare stability study protocol as per attachments -V (Stability Study Protocol) and get it issued before final dispatch of subjected batch.
Samples required for stability studies are taken by IPQA supervisors on packing line and sent to QC department.
Sample quantity of any subjected batch contains samples for following requirements:
- Sample required for one complete analysis for each time point.
- Total quantity for all time points.
- One additional pack for any unforeseen event like OOS.
- Placebo sample for each time point analysis.
Charging of samples:
The samples are charged into the stability chamber after putting the sticker labels on each pack within 7 days of finished product analysis completion.
Consider Initial analysis of finish product as initial time-point /initial analysis results. In case оf delay of more than one month in FP analysis and stability charging, perform again an analysis to consider it as initial time-point analysis of stability study (Provided that delay in charging is justified).
Due dates of analysis are indicated in the Stability Studies Calendar (Attachment-III).
Update stability calendar soon as new sample placed in stability chamber for studies (in electronic form). Take print out of updated calendar in every six month and review it for completeness.
Prepare monthly calendar for withdrawal of sample and get it reviewed before every month start.
Withdrawal of samples:
As per the calendar the samples are withdrawn within 5 days of the due date of analysis for Long Term and Intermediate Stability Studies and 3 days of the due date of analysis for Accelerated Stability Studies. Samples are withdrawn from the chambers according to monthly record of Stability Samples Withdrawal (Attachment-IV).
QC Chemist registers samples in the Stability Samples Register (Attachment-V) and Stability Samples Maintenance Record (Attachment-VI). The Stability Samples Maintenance Record is updated every six months.
Analyses of samples
QC Chemist analyzes sample as per approved Finished Product Standard Test Procedure during 15 days since its withdrawal day. When performing test keep samples at room temperature and in specially designed areas.
Note:
- Number of injections for Assay is the following: blank solution (1), standard solution (6), test solution (2).
- Number of injections for Related Substances is the following: blank solution (1), placebo solution (1), standard solution (3), test solution (2).
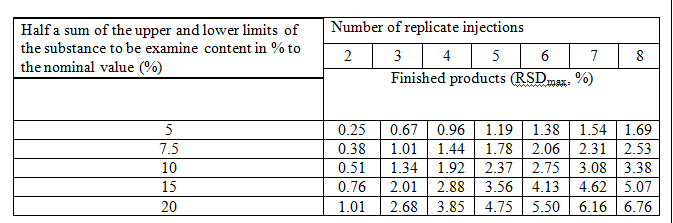
- RSDmax criteria for Assay for Chromatographic System Suitability is described in the table.
Result processing
Test results of the analysis are entered in the analytical reports and COA is prepared. Laboratory In-charge checks the documents and sends the same to Head QC for approval. QC Chemist enters all data in the corresponding Stability Study Report (Refer Attachment – VI).
Any change noticed in the product during stability studies must be immediately reported to QC Head for future action plan.
On completion of stability studies, the samples from the stability chamber shall be destroyed.
Collection of Sample:
For each new product collection of sample shall be done for first three consecutive batches for Stability study under Accelerated temperature and humidity conditions.
For all products, at least one batch per year shall be kept under Long-term stability study.
Quality Assurance personnel shall collect the sample as per instructions from Head Quality Assurance as per stability study schedule
Sample Size:
Samples shall be collected as intact-marketed packs. The quantity of sample collected shall be insufficient number depending upon the product and its stability indicating tests.
Storage of Sample:
Samples shall be kept at Controlled temperature (25 + 2 0C) and Relative humidity (60 ± 5%) conditions for Long-term stability study.
Samples shall be kept at temperature (40 + 20C) and Relative humidity (75 + 5%) for Accelerated stability study.
Testing Parameter and Periodicity of Analysis:
Testing parameters for different products shall be as per the Stability program mention in stability study protocol.
The initial analysis data shall be taken from Certificate of analysis of the respective product and batch, provided the Date-in (To) of stability study sample is within the period of one month from the date of release of the batch. In case the period is more than one month the initial analysis shall be carried out once again at the time of “Date-in”.
If the stability assay method is different from the product release assay method, then initial analysis shall be performed as To as per stability assay method.
Additional stability indicating tests (other than product release specification) if any, as per stability program, shall be performed at the time of “Date-in” (To).
Periodicity of analysis for stability samples shall be as follow:
- a) Accelerated stability conditions (400C ±20C/75%±5%) = 0, 03 and 06 months.
- b) Long term Stability Conditions (250C±20C/60%±5%) = 0,03,06,09,12,18,24 36 months.
- c) Long term stability study shall be carried out upto the expiry period of the product.
Long-term stability analysis shall be performed within ±7 working days of the due date. Accelerated stability samples shall be analyzed within ± 5 working days of the due date.
The sample for stability studies shall be taken within one month from the date of approval of the batch. An annual schedule of the batches to be studied for stability and the date of analysis shall be made every December for the following year.
Entry in stability register shall be made accordingly.
Evaluation of Stability Data:
Evaluation of stability data shall be made once in a year. Based on evaluation of data, recommendations if any shall be made by Head Quality Assurance.
During stability study any adverse change in physical parameters, pH, and failure in assay, content uniformity, and dissolution rate shall be brought to the attention of Manager Quality Control and QA Head.
Investigation shall be done on the affected batch along with all the batches produced at the same time. Investigation shall be extended to all ingredients used, manufacturing process, process parameters followed and analysis at different stages.
Decision on recalling the affected batches and /or along with other batches produced during the same time shall be taken by Head QA in consultation with Managing Director and Chairman.
REFERENCE:
In-house
ABBREVIATIONS:
SOP: Standard operating procedure
QA: Quality assurance
QC: Quality control
Dept.: Department
DISTRIBUTION LIST:
Quality Control Department
Quality Assurance Department
HISTORY OF REVISION:
| Version Number | Effective Date | Reason for Revision |
| 00 | – | New SOP |
Attachment-I
| Shelf 1 | ||
| Shelf | ||
| Shelf 4 | ||
Attachment-II
|
STABILITY SAMPLE SCHEDULE |
|||||||||||||||
| Sr. No. | Product Name | Batch no. | Date in | Acc | Long | 40ºC ±2ºC/75%±5%RH | 30ºC ±2ºC/75%±5%RH | ||||||||
Attachment-III
MONTHLY RECORD OF STABILITY SAMPLES WITHDRAWAL
| Name of Product |
Batch No. | Quanitity |
3M |
6M |
9M |
12M |
18M |
Sample withdraw by | Received By | Remarks |
|
|
|
Attachment-IV
STABILITY SAMPLES REGISTER (Long Term)
| Laboratory logbook (Stability samples) | ||||||||||||||||||||
| Withdrawal date
|
Name of Product
|
Batch No. | Type of pack
|
Mfg. date
|
Exp. date
|
Station
|
A.R.No.
|
Analysed by | Date completed | Remarks | ||||||||||
|
|
|
|||||||||||||||||||
Attachment-V
STABILITY SAMPLES MAINTENANCE RECORD
| Stability Samples Maintenance Record | ||||||||||||||||
| Product name | Batch No. | Acc. | Initial | 3М | 6М | Real time | 3М | 6М | 9М | 12М | 18М | 24М | 36М | 48М | 60М | Remarks |
Attachment-VI
| Accelerated Study Stability Data
Generic Name: Product Name- |
Batch no.: | ||
| Label claim / Composition: | Mfg. Date: | ||
| Packing: – | Purposed Exp. Date: | ||
| Sample Qty. : 20 Tablets | Date of initiation: | ||
| Frequency of Testing | Initial, 1, 2, 3 & 6 months | Date of Completion: | |
| Storage Condition: At 40° ± 2°C & 75% ± 5% RH | Ref. Protocol: | Batch Size: | |
| TESTS | LIMITS | RESULTS | ||||||||||
| INITIAL | 1 MONTH | 2 MONTH | 3 MONTH | 6 MONTH | ||||||||
| Description. | ||||||||||||
| Average weight | ||||||||||||
| Uniformity of weight | ||||||||||||
| Identification | ||||||||||||
| Disintegration time | ||||||||||||
| Dissolution | ||||||||||||
| Related Substance | ||||||||||||
| Assay : | ||||||||||||
| Long term Stability Data
Generic Name: Product Name: |
Batch no.: | |||
| Label claim / Composition: | ||||
| Packing: | Purposed Exp Date: | |||
| Sample Qty. : 20 Tablets | Date of initiation: | |||
| Frequency of Testing | Initial, 3, 6, 9,12, 18 & 24 months | Purposed Date of Completion: 3 | ||
| Storage Condition: At 30° ± 2°C & 65% ± 5% RH | Ref. Protocol: | Batch Size: | ||
| Tests | LIMITS | Results | ||||||||||||||
| Initial | 3 Month | 6 Month | 9 Month | 12 Month | 18 Month | |||||||||||
| Description. | ||||||||||||||||
| Average weight | ||||||||||||||||
| Uniformity of weight | ||||||||||||||||
| Identification | ||||||||||||||||
| Disintegration time | ||||||||||||||||
| Dissolution | ||||||||||||||||
| Assay : | ||||||||||||||||