STERILITY ASSURANCE 〈1211〉
INTRODUCTION
Definition of sterility – An item is deemed sterile only when it contains no viable microorganisms.
However, this textual definition cannot be applied to actual items labeled as sterile because of irresolvable limitations in testing. Sterility cannot be demonstrated without the destructive testing of every sterile unit.
In a real sense, microbiological safety is achieved through the implementation of interrelated controls that in combination provide confidence that the items are suitable for use as labeled. It is the controls that provide the desired assurance from microbiological risk rather than the results of any in-process or finished goods testing. The verification of safety of products labeled sterile is generally known as “sterility assurance”.
The establishment of an effective sterility assurance program requires information about the material to be sterilized.
An initial determination should be made regarding the potential for terminal sterilization of the material in its primary container applying the principles defined in Sterilization of Compendial Articles 〈1229〉. As described, the appropriate process provides a balance between conditions that are lethal to potential bioburden present in/on the item and those that preserve its essential quality attributes.
Depending upon the results of that determination, sterility of the item may be achieved by either aseptic processing or terminal sterilization.
The potential for a process that relies on both the protective measures inherent to aseptic processing and the lethal nature of terminal sterilization may offer advantages.
- Regardless of the process selection decision,
- the establishment of design,
- operation,
- process controls, and
- monitoring systems
is essential to provide the necessary confidence in the outcome.
The production of sterile products is subject to numerous factors that influence the outcome (see Figure 1). The identified factors in the image should be considered for their impact on sterility of the final product, although not all of the influencing elements are depicted.
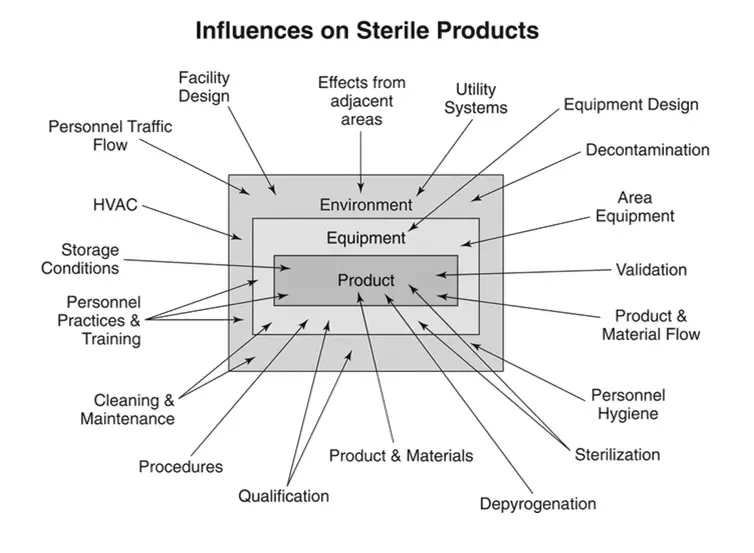
Figure 1. Influences on sterile products.
The decisions made relative to the influences shown in Figure 1 will determine the success of the sterility assurance program. Poor choices, regardless of any successful process controls associated with them, must be acknowledged as fundamentally unsound.
The process design objective is related to contamination controls intended to obviate risk of microbial ingress. This focus is appropriate regardless of whether the process used is aseptic processing or terminal sterilization.
Recognition that operating personnel are the most significant contributors of microbiological risk leads to design preferences and operating principles that should be adhered to with respect to sterile operations.
This knowledge underscores the importance of separating personnel from the aseptic environment and limiting their interaction with sterilized components and product(s).
The means for accomplishing these goals are embodied in two complementary practices (1):
- The use of automation technology—to reduce or eliminate personnel interventions and thus personnel-borne contamination
- The use of separative technologies—to eliminate, to the extent technically possible, human sourced contamination
Thus the implementation of appropriate contamination control procedures is paramount in design and operation of sterile product manufacturing systems.
Consideration of these principles adapts the Quality by Design (QbD) approach widely adopted in regulatory standards .
Using QbD concepts in sterile operations is markedly different from the applications in the typical formulation, pharmaceutical, chemical, or biological synthesis process.
The establishment of direct linkage between a monitored condition and process outcome with respect to sterile manufacturing is statistically difficult and analytically uncertain.
The situation with respect to the definition of physical design elements is similar. Given the great variation in sterile product manufacturing with respect to scale, configuration, and complexity, it follows that the design alternatives and operating practices must also be flexible. Thus, the recommendations provided in this chapter are entirely non-numeric, because there are no ready means with which to demonstrate the suitability of specific values.
Instead, QbD for sterile processing should be driven toward a singular goal of optimizing contamination control with a particular focus on the microbial risk impact of personnel. The specific means vary but should be of prime consideration in process design. Figure 2 outlines the elements that contribute to sterility assurance, as described
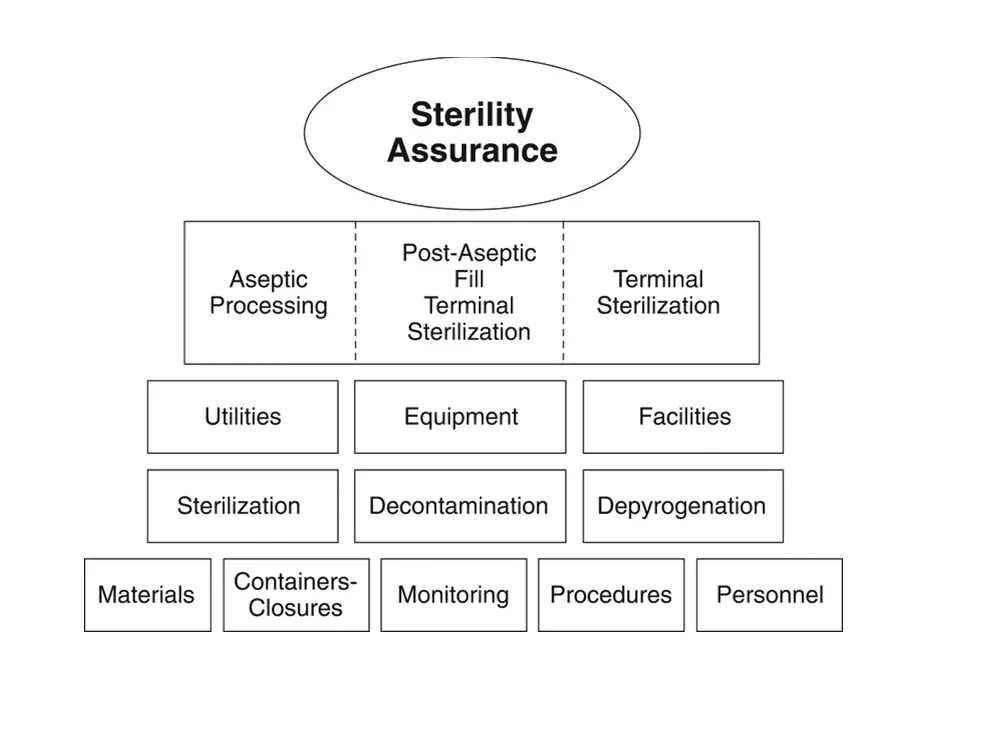
Figure 2. Elements contributing to sterility assurance
Aseptic Processing
There are a substantial number of sterile products that cannot be terminally sterilized because of adverse impact on the product/package’s essential properties and must be prepared by aseptic processing.
Aseptic processes are designed to prevent the introduction of viable microorganisms into/onto separately sterilized materials during their assembly into a sealed sterile package.
Aseptic processes can vary in complexity from comparatively simple filling/sealing to challenging and lengthy manufacturing sequences required for complex items. Regardless of process scale, all of the individually sterilized materials must be protected from contamination from the point of sterilization through closure of the primary package.
This is accomplished through adherence to the principles described below in which an International Organization for Standardization (ISO) 5 condition is maintained when materials are exposed to the environment (see Microbiological Control and Monitoring of Aseptic Processing Environments 〈1116〉). Exclusive to aseptic processing is the execution of process simulations that support batch or campaign duration.
Terminal Sterilization
Terminally sterilized products are the lowest risk category of sterile pharmaceutical products. Unlike products aseptically manufactured under conditions designed to prevent microbial ingress, terminally sterilized products are subjected to a sterilization process that imparts a quantifiable safety level. Terminal sterilization processes achieve this by delivering measurable physical conditions that correspond to microbial lethality. For terminally sterilized products, sterility assurance is defined in terms of the probability of non-sterility (PNS), or the probability of the terminal sterilization process generating a nonsterile unit (PNSU). Terminal sterilization processes must achieve a consistent validated performance of a PNSU of ≤10−6 (a probability of NMT 1 nonsterile unit in 1 million units produced) (see 〈1229〉). The convention by which terminal sterilization cycles are developed and validated ensures that the actual PNSU is typically much lower (better) than the minimum standard of <10−6.
Chapter 〈1229〉 summarizes the common requirements for sterilization process design, development, validation, and process control. Terminal sterilization processes share common requirements of well-defined process parameters strictly controlled within defined operating limits. Terminal sterilization must be supported by a system of product disposition, which includes the assessment of critical physical process parameters, pre-sterilization product parameters (e.g., bioburden, container–closure integrity), and environmental parameters. Terminal sterilization can rely on parametric release practices to obviate the need for sterility testing (see Terminally Sterilized Pharmaceutical Products—Parametric Release 〈1222〉).
Post-Aseptic Processing Terminal Sterilization
An aseptic process followed by a terminal sterilization process provides superior control over the pre-sterilization bioburden, such that the subsequent sterilization process can be designed with less overall lethality, thereby making it possible to substantially extend the use of terminal sterilization to products with greater sensitivity to the applied energy of the process. From a patient safety perspective, this approach has the following distinct advantages:
- An adventitious contaminant introduced during aseptic processing is easily killed by the terminal sterilization step, reducing the extent of in-process environmental monitoring performed
- Bioburden controls for the terminal process are simplified because all units have been aseptically filled
- Where a product is made using either process alone, the limitations of each (no terminal lethal component in aseptic, more degradation in terminal sterilization) would persist
- Where bioburden is controlled through aseptic processing, terminal sterilization can be applicable at lower lethality levels.
Classical F0, time-temperature, and radiation dose (kGy) targets for sterilization processes are arbitrarily selected and intended to simplify process validation, but in reality serve to reduce the use of terminal treatments. Physical lethality data based on fixed numerical values are inherently conservative and disregard the degradative impact of the sterilization process on the product. The focus must be on the ability of the process to kill bioburden organisms rather than biological indicators (see 〈1229〉 and Moist Heat Sterilization of Aqueous Liquids 〈1229.2〉).
While product quality attributes can be impaired by “standard” sterilizing conditions, the combined process can utilize less aggressive sterilizing conditions to minimize adverse effects upon the product and primary packaging materials. If the terminal treatment follows aseptic processing, then the sterilizing conditions need not be excessive as there is essentially no risk from pre-sterilization bioburden in the filled containers. Sterilization process conditions would be dictated by the specifics of the product in parallel with the establishment of appropriate controls on pre-sterilization bioburden derived from environmental and prefiltration isolates.
Containers and Closures
The container and closure for a sterile formulation are integral parts of the sterile product. The container materials provide essential protection to the product throughout its shelf life and are chosen to minimize interaction with, preserve the quality attributes of, and facilitate dispensing of the sterile product. The container materials should be readily sterilizable, either separately prior to filling and/or together with the formulation in a terminal process. Containers and closures should be selected for:
- Reliability of container–closure integrity over the shelf life
- Absence of interaction with formulation materials
- Ease of handling in the processing environment and during administration
- Tolerance of variation in equipment and other components
- Cleanliness including freedom from particulates and absence of leachable or extractable chemicals
- Compatibility with the product
- Control over endotoxin content (where appropriate)
- Protection of components prior to use (where appropriate)
- Compatibility with preparation, sterilization, and depyrogenation processes (where appropriate)
The essential aspects of container–closure materials for sterile products are subject to numerous requirements elsewhere within USP–NF. The reader must consider the content provided in Injections and Implanted Drug Products 〈1〉, Bacterial Endotoxins Test 〈85〉, Visible Particulates in Injections 〈790〉, Package Integrity Evaluation—Sterile Products 〈1207〉, Depyrogenation 〈1228〉, and 〈1229〉.
Decontamination
Decontamination is a broadly defined term used to describe a variety of processes that reduce microbial populations without an expectation for total kill.1 It is not a substitute for sterilization; a sterilization process should be used wherever possible. A variety of chemical agents and methods are used that vary depending upon the application. Decontamination is used for bioburden reduction of materials, equipment, and environments in support of sterile product manufacture:
- For materials and surfaces that cannot be sterilized
- For materials and surfaces that do not require sterilization
Decontamination processes are ordinarily separated into two major categories based upon their effectiveness against spore-forming microorganisms (see Disinfectants and Antiseptics 〈1072〉). Sporicidal treatments are used in critical applications such as isolator decontamination, air-lock/pass-throughs, etc. Their toxicity to personnel and sometimes corrosive chemistry may preclude their exclusive use for microbial control. Non-sporicidal agents have fewer safety and material impact concerns, and the occasional use of a sporicidal agent is required to control spore populations. Applications for decontamination are diverse; among the more common uses are:
Decontamination of controlled environments and non-product contact surfaces
- In conventional cleanrooms, including restricted access barrier systems (RABS), this is predominantly a manual process performed after cleaning of the room/production line
- Decontamination of items upon transition into an environment of higher classification
- Isolators commonly use an automated process
- Periodic decontamination of operator gloves during processing
Decontamination of product contact surfaces
- Large equipment (e.g., stopper bowls) can be manually sanitized on a frequent basis in addition to sterilization to avoid the extensive manipulation required for their installation post-sterilization2
- Re-decontamination of sterilized equipment after aseptic assembly or intervention
- Periodic decontamination of previously sterilized utensils prior to interventions
Depyrogenation
The minimization of pyrogen content is a requirement for injectable products. During the production of sterile products, depyrogenation processes are used in a variety of ways to minimize pyrogenic contamination of surfaces, materials, and products. Details on depyrogenation processes are provided in 〈1228〉.
Equipment
Equipment used for sterile product manufacturing varies in its impact on the manufacturing process and on product quality and should have several important characteristics. For example, the equipment should:
- Operate reliably and produce products of consistent quality
- Not adversely impact essential product quality attributes
- Be easily cleanable and sterilizable, as necessary
- Minimize human intervention during set-up and operation through such features as physical separation, automation, and robotics
- Be tolerant of variations in container–closure materials
- Designed to minimize product exposure to the background environment
The extent to which the equipment interacts with process materials and the product affects the level of impact. Process equipment can influence the quality of the finished product in a variety of ways, and this can occur prior to and after sterilization and depyrogenation.
equipment in direct contact with components, containers, closures, and sterile products
This equipment category includes those items in direct contact with the drug substance, drug product, raw materials, and primary packaging components, including, for example, mixing and storage vessels, piping systems and tubing, filters, filling pumps, lyophilizer shelves, and feed hoppers. Product contact surfaces of this equipment are designed and may require additional treatment to minimize adverse impact (microbial, particulate, and chemical) on the contacted materials. The procedures used for the cleaning and sterilization of direct contact surfaces, including dirty, clean, and sterile hold times, must be validated to ensure they do not adversely impact essential product quality attributes as well as to verify the effectiveness of the cleaning procedure and that no microbial recontamination/proliferation occurs during equipment storage. Direct contact utensils are subject to the same considerations. With appropriate consideration of materials’ compatibility, single-use disposable equipment (supplied sterile when necessary) may be utilized.
equipment having indirect contact with components, containers, closures, and sterile products
Equipment having a significant impact on product quality, that does not contact components, primary packaging materials, and sterile products, includes the electro-mechanical elements (non-product contact) of filling machines, stoppering machines, and sterilizers. The performance of this equipment can change fill weight, particle size, moisture level, content uniformity, container–closure integrity, and other essential quality attributes. This equipment is ordinarily located near exposed product contact equipment surfaces. For example, a pre-assembled filling set (product contact equipment) may be installed on a filling machine (significant impact without product contact), which provides control over the fill volume or weight. The surfaces of this equipment must be compatible with the cleaning and microbial decontamination and/or sterilization agents employed.
other equipment
Some equipment has only an indirect impact on product quality, for example, conveyors, turntables, balances, air samplers, and carts. The influence of this equipment is largely on the environments in which the product is made. The exposed surfaces of this equipment must be compatible with the cleaning and decontamination agents used.
Facilities
Sterile manufacturing operations are supported by administrative, laboratory, maintenance, and warehouse functions and other activities. The impact of these operations on the location and overall design of the sterile manufacturing area must be considered. Emphasis should be given in facility design to the flows of materials, components, personnel, equipment, and waste streams throughout the facility and to the orderly transition of items between environments of different classifications to prevent mix-up and avoid product contamination. Facility environmental and utility systems must be designed to minimize microbial, chemical, and particulate contamination. The facility design must be supported by practices and procedures such as cleaning and decontamination, gowning, and material transfer. The architectural details of the facility infrastructure must consider the means for cleaning and disinfection. Detailed design recommendations can be found in the International Society for Pharmaceutical Engineering’s Baseline Guide: Sterile Product Manufacturing Facilities (7).
The core activities for sterile product manufacture are carried out in classified environments operating in conformance with the ISO 14644 series of standards (8). A pressure cascade descending from the more critical areas to less critical is commonplace. In general, the more protection that materials have from potential sources of contamination during holding or processing, the less impact the facility has on the process outcome. Human operators within ISO 14644 classified cleanrooms used in aseptic processing are the greatest risk to product safety; therefore, no single risk mitigation factor in aseptic processing is more important than minimizing risk emanating from gowned operators.
Early-stage container–closure and equipment washing and preparation are carried out in lower classification areas (ISO 7–8). Nonsterile formulation is typically carried out in ISO 6–7 environments. The production materials are introduced into the processing area where subsequent steps are performed. The bioburden level of the materials influences the detailed design of the facility and its controls. Nonsterile materials (e.g., formulation, containers, closures, equipment, and utensils) require subsequent sterilization and, where necessary, depyrogenation. Sterile materials are introduced through airlocks and pass-throughs. The facility design controls for the background, and processing environments should be chosen to preserve the intended microbial attributes of the in-process and finished materials.
Table 1 provides some examples of formulation and filling environments.
Table 1. Examples of Environments for Processing
| Processing Technology | Background Environment | Processing Environment | Reference |
| Conventional cleanroom | ISO 5–7 | ISO 5 | FDA AP 2004 (9), EMA Annex (10) |
| Closed RABS | ISO 7 | ISO 5 | ISPE (11), PHSS (12) |
| Open RABS | ISO 5 | ISO 5 | ISPE (11), PHSS (12) |
| Closed isolators | CNCa | ISO 5 | FDA AP 2004 (9), PDA TR No. 34 (13), ISPE (7) |
| Open isolators | CNC-ISO 8 | ISO 5 | FDA AP 2004 (9), PIC/S (14), PDA TR No. 34 (13), ISPE (7) |
| Blow-fill-seal/form-fill-seal | ISO 5–7 | ISO 5 | FDA AP 2004 (9), Baseman (15) |
| Closed systems | CNC | Not applicableb | PDA TR No. 28 (5); Agalloco, Hussong, et al. (16) |
| Terminal sterilization | ISO 8 | ISO 5–7 | EMA Annex (10) |
a Controlled non-classified—a non-classified controlled environment with filtered air supply.
b As the process occurs with a closed system, there is no separate processing environment.
[Note—Table values represent the operational condition and are adapted from the reference documents.]
conventional cleanroom
The critical activities are performed in ISO 5 environments supported by surrounding ISO 5–7 environments where gowned personnel are normally located. There may be only limited separation between gowned personnel and sterile materials and product contact surfaces. The critical activities are performed within a unidirectional airflow environment. Decontamination of the cleanroom is commonly performed by personnel.
restricted access barrier systems
The typical RABS provides ISO 5 unidirectional air within the barrier and is situated in a conventional ISO 5–7 cleanroom. RABS may be designed to allow for opening of barriers to enable human intervention, or they may be designed to operate closed with the same operational restrictions regarding operator access that applies to isolators (see below). Air overspill from within the barrier is designed to prevent the ingress of contamination. Operator manipulation of sterile items is achieved using glove ports, and material transfers are accomplished without opening the system. A RABS that is opened mid-process should be treated as a conventional cleanroom (see above). RABSs require decontamination prior to use. This may be accomplished either manually or using automated systems.
isolators
Isolators provide complete separation between personnel and the enclosed ISO 5 processing environment. A defined pressure differential is maintained between the ISO 5 environment and the surrounding area. Air overspill provides an aerodynamic seal at points where the product exits the isolator into an external environment of lesser classification or no classification. The use of unidirectional air is not required in isolators. Isolators are commonly decontaminated using automated systems.
blow-fill-seal and form-fill-seal
These technologies form, fill, and seal flexible walled containers in an ISO 5 environment. Blow-fill-seal (BFS) and form-fill-seal (FFS) equipment configurations allow for installation in a variety of background environments. The critical activities are performed within a unidirectional airflow environment. Decontamination is performed as is common for the background environment.
closed systems
These systems provide for complete separation of production materials from personnel and surrounding environment. Closed systems can be single- or multiple-use vessels/chambers with means for materials ingress/egress. The designs avoid any human interaction or environmental contact with sterile materials. These systems vary in complexity and are sterilized either in situ or prior to use.
terminal sterilization
Filling systems and environments for containers to be terminally sterilized can be accomplished in ISO 7 or better environments. The critical activities are performed within a unidirectional airflow environment. Decontamination of the cleanroom is commonly performed by personnel.
Materials (Active Pharmaceutical Ingredients, Excipients, and Process Aids)
The preparation of sterile products encompass a wide range of materials including active pharmaceutical ingredients (small and large molecules), excipients, solvents (usually water), process gases and processing aids, all of which contribute to the microbiological quality attributes of the product. Depending upon the product being manufactured, this can require consideration of bacterial, endotoxin, and particulate contamination. Specific microbiological quality testing requirements for inactive and active ingredients testing is often specified in a relevant USP–NF monograph. Requirements for microbiological testing for total aerobic bacteria, yeast and mold counts, and specified organisms are given in Microbial Enumeration Tests 〈61〉 and Tests For Specified Microorganisms 〈62〉, and the recommended but non-mandatory enumeration targets for microbiological testing are given in Microbiological Examination of Nonsterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use 〈1111〉. Some specific products may require other testing for specified organisms, including viruses, as a requirement of their regulatory approval. Requirements for bacterial endotoxin testing in injectable products are covered in 〈85〉, which describes qualification of Limulus amebocyte lysate testing for acceptable levels of bacterial endotoxin. Raw material specifications must be appropriate to ensure that the manufacturing process consistently results, as demonstrated through process validation, in products conforming to the microbiological critical quality attributes.
Microbial contamination may be present on/in active pharmaceutical ingredients, excipients, and primary packaging materials. Controlling bioburden in materials and formulated product is a critical aspect of sterility assurance. The nature of the active pharmaceutical ingredient, the excipients, and the compounded product intended to be sterile are critical elements of the product knowledge. Recognizing the potential impact on critical quality attributes, the microbiological attributes of materials should be controlled through adequate supplier controls, shipping, receipt, sampling, handling, and storage. These controls should be commensurate with the microbiological risks to process controls and sterile product safety. Laboratory results should not be utilized to rationalize inadequate process controls on the part of the supplier.
The potential impact of the materials on the microbiological critical quality attributes of presterilized and finished product must be assessed by evaluating them with appropriate compendial assays or validated alternatives. Risk assessment should consider the origin of the material (e.g., fermentation, chemical synthesis, biologically derived, enzymatic, semi-synthetic synthesis, natural origin). Materials of biological origin may have higher inherent microbiological risk than materials derived solely from chemical synthesis. Materials may have inherent physicochemical properties that mitigate microbiological risks (e.g., low water activity, extreme pH, inherent antimicrobial properties) or increase microbiological risks (e.g., aqueous solution, growth supportive nutrients); this risk of supporting microbial proliferation must be assessed.
Monitoring
Environmental monitoring is employed to qualitatively assess the effectiveness of the design and operational controls to provide suitable facility hygiene. It is neither a substitute for good facility, equipment, and process design, nor compensation for deficient practices and behavior. There are inherent limitations with all forms of viable and non-viable monitoring in terms of sample size, sample location, and recovery capability that preclude their use as anything more than an indication that a facility is operating within an acceptable state of control. Monitoring provides only a snapshot in time of the actual environmental conditions and excessive sampling due to its potentially intrusive nature can actually impair product safety or generate counts unrelated to process performance by increasing activity proximate to the critical zone.
Independent of air, surface, and personnel monitoring, media fills and sterility testing provide additional (albeit analytically and statistically limited) means to evaluate the robustness of the cleanroom design, performance, and effectiveness of cleaning/decontamination procedures, personnel gowning integrity, and aseptic practices.
Environmental control can be measured only by the monitoring performed. Satisfactory monitoring performance is the result of proper design and operation as described in this chapter and not a means to establish that condition. Performance criteria are established according to the classification of the room and its usage (see 〈1116〉).
viable monitoring
Viable monitoring consists of detecting and estimating the level of culturable microorganisms in the air, on surfaces, and on personnel. Sampling locations are defined following a risk assessment and sampling is executed by trained operators using a variety of methods including:
- Active air sampling
- Passive air sampling
- Viable particle counting using fluorescence technology
- Contact-plate sampling of surfaces, gloves, and gowns
- Swabbing of surfaces
- Personnel monitoring
non-viable monitoring
Non-viable monitoring measures the number and size of particulates present in the air. It can be used to initially classify the cleanroom in accordance with ISO 14644-1 and to assess routine manufacturing conditions (8). When used for the purposes of monitoring, it can be performed under static conditions (no activity) and/or dynamic conditions (routine operation). Non-viable particle monitoring is performed using calibrated particle counters.
media fills/aseptic process simulations
Process simulations are exercises in which the performance of an aseptic activity is evaluated using a sterile growth medium. The medium can be directly substituted for the product or added to it. Aseptic process simulations are typically performed before the introduction of new or revised process components (e.g., products, facilities, equipment, personnel, containers and closures, and processes) and periodically thereafter (17). Process simulations should be fully representative of processing conditions and activities utilized during routine production.
sterility testing
The sterility test is a harmonized compendial test. It must be understood that while execution of the test is required for the release of sterile products where parametric release has not been approved, it cannot prove the sterility of the materials tested. It should be recognized that parametric release is the default mode of sterile product release.
Personnel
Personnel play an essential role in the preparation of sterile products. The essential activities they perform include cleaning, assembly, equipment operation, material transfer, environmental monitoring, and decontamination. While personnel are often necessary for the performance of these activities, the contamination derived from them must be prevented from entering the production materials before and after sterilization. The importance of the controls necessary to minimize exposure to and the release of human microbial contaminants in a sterile product manufacturing environment cannot be overstated.
The personnel involved in the preparation of sterile products must:
- Understand the principles of microbiology, sterilization/depyrogenation, aseptic processing, and contamination control
- Be proficient and diligent in gowning practices. Personnel required to wear aseptic gowning should periodically demonstrate their ability to properly gown
- Adhere to proper aseptic technique during all aseptic activities even when these are performed in a RABS or isolator. Periodic demonstration of these skills can be beneficial
- Be familiar with and adhere to standard operating procedures
- Practice good personal hygiene to minimize contamination potential
- Be trained in the proper and safe operation of necessary equipment
- Be monitored microbiologically after performing aseptic operations
Procedures
Written procedures define the operations that have been determined through validation studies and experience to be effective in controlling and facilitating the manufacture and quality of pharmaceuticals and biopharmaceuticals. Procedures are especially important for the critical processes designed to assure the sterility of terminally sterilized and aseptically produced drug products. Procedures should be periodically reviewed and evaluated to ensure they are effective and current.
interventions and intervention procedures
There are two types of interventions associated with the aseptic production of sterile drug products. Inherent interventions are those activities that are an integral part of the aseptic process and are performed during the production of every batch. They include set-up, replenishment of components, weight and volume checks and adjustments, and environmental monitoring. Corrective interventions are those activities that correct problems and might not be performed during the production of every batch. They can be minimized and should be avoided through careful process design. Examples include stopper jams, broken and fallen glass, defective container seals, liquid leaks, and mechanical failures requiring manual correction.
Each intervention, whether inherent or corrective, should be covered by written procedures sufficiently detailed to enable personnel to perform the intervention correctly, and to perform the intervention the same way each time regardless of whomever performs it. For example, procedures should specify the number of units, their locations, and how the units are to be removed, and personnel must be trained so they can correctly execute the procedures. No intervention should be permitted for which there is not a defined procedure. The following concepts should be considered during development, review, and implementation of intervention procedures.
- Interventions performed during all forms of processing must be recognized as increasing the risk of contamination dissemination and are to be avoided or designed out of the process to the extent possible
- Procedures for interventions should be critically reviewed to eliminate and/or simplify aseptic processes by reducing the frequency of inherent interventions and making all interventions easier to perform
- Interventions should be designed for minimal risk of contaminating sterile and nonsterile materials
- All interventions should be performed using sterilized tools whenever possible
- Intervention procedures should be established in detail for all inherent interventions, and more broadly for corrective interventions (where some flexibility is necessary due to greater diversity)
- Interventions should be incorporated in periodic media fills to evaluate the aseptic practices of the operators
Operators should initially, and periodically thereafter, be trained in all of the procedures they are expected to perform. Considerations for operator activity during the non-aseptic filling of containers should parallel those described above to minimize the potential for contamination ingress with somewhat less rigor than those needed for aseptic operations.
Sterilization
The most effective means for the control of microbial population is sterilization, a process that either kills or removes viable microorganisms. In the production of sterile products, sterilization processes are used to prevent microbial contamination. Terminal sterilization processes that reproducibly destroy microorganisms in the final product container are the preferred means for the production of sterile products. Sterile products that cannot be terminally sterilized rely on individual sterilization processes (e.g., steam, radiation gas, filtration) for the various materials that comprise an aseptically processed sterile product. In addition, sterilization processes are used for product contact and other non-product contact items used in a variety of applications during the preparation of sterile products to provide absolute control of bioburden. Details on sterilization processes are provided in 〈1229〉.
Utilities
The manufacture of sterile products requires utilities that can have a substantial impact on the final product. Some of the utilities in the facility can become an integral part of the formulated product (e.g., Water for Injection, Nitrogen) and appropriate design of the production and distribution system for these is essential. The systems for these are tightly controlled and frequent monitoring of the utilities produced is customary. These utilities may be also used in the process, and not become a part of the sterile product. Other utilities (e.g., clean steam, compressed air, Purified Water) that are used in the cleaning/decontamination of facilities, and/or preparation of equipment, containers, and closures can also be subjected to microbial control.
Utilities included in the product, in direct product contact, and in the preparation of equipment, containers, closures, and other items must meet the requirements defined in the appropriate USP–NF monograph. The systems for their preparation should be subject to formalized controls that maintain a controlled state over time. This is accomplished through a number of related practices essential for continued use of the system over an extended time. The essential practices to maintain controlled status of the utility systems include: calibration, change control, corrective and preventive maintenance, and ongoing process control.
There are other less impactful utilities (e.g., vacuum, cooling water) necessary for the operation of the facility and equipment. Although these non-product contact utilities may lack monograph requirements, their reliable operation is necessary for consistent production of sterile products.
SUMMARY
The safety of products labeled sterile requires that their critical quality attributes consistently meet specifications. Sterility is the most essential quality attribute. Sterility is an unqualified concept in which an item is devoid of living microorganisms capable of reproduction. Monitoring of all types, environmental (viable and non-viable; air, surface, and personnel), media fills, and sterility tests are forms of microbiological analysis that have been historically employed as proof of “sterility”. These assessment tools cannot provide definitive evidence of either “sterility” or “nonsterility”, because the means to confirm either of those conditions non-destructively is not scientifically possible. Confidence in sterile product manufacturing is realizable only by a holistic approach in which all of the supportive elements of the operations are given due consideration and emphasis (17).
The absolute nature of sterility presents the practitioner with an inherent paradox—there is no ready means to demonstrate sterility of an item in the absolute sense regardless of the means used to provide it. Test methods including those defined in this compendium (Sterility Tests 〈71〉) utilize a number of samples taken from a large population to infer the “sterility” of the whole. Sterilization procedures including those validated for parametric release can deliver a low probability of a nonsterile unit, but not absolute assurance that “sterility” actually exists.
The uncertainty associated with proof of “sterility” notwithstanding, the means by which sterility assurance is provided are reasonably well defined. The “sterility” of any item is definitively established by the process controls summarized in this chapter rather than any form of monitoring or sampling. For terminally sterilized products, greater weight can be placed on the sterilization process utilized than on any form of testing. Confidence in aseptic processing is a result of sound design, reliable equipment, quality materials, effective procedures (including supportive sterilization processes), and personnel proficiency rather than through sampling dependent sterility testing, microbiological monitoring, and process simulation.