Cleaning process Control
To verify and qualify a cleaning process, the cleaning process needs to be unremarkable and sufficiently robust for the to-be-cleaned equipment. It would be clear which way are considered part of the product process /unit operation and which are part of the cleaning process, for Illustration if the pre-rinse or wash-out which may be routinely applied to bring the outfit in a good starting position is part of the overall cleaning process or not.
Another illustration is the cleaning of chromatography columns, which are generally cleaned with buffers previous to
the chromatography descent cleaning.
To assure repetition and robustness of the cleaning, acceptable cleaning instructions are needed.
For inhouse manual cleaning, this is generally fulfilled by sufficiently detailed cleaning instructions, including an unequivocal description of the attributes to be used and how to handle these, together with acceptable training.
The detailed description should consider
1. the system boundaries
2. drawing agents/ detergents to be used
3. volumes and or attention
4. influx or wash times, and temperatures
5. the sequence of drawing way or pre-defined procedure
6. in process analyses
7. description of pumps used (if demanded)
For automated cleanings this should be assured by the outfit design together with the cleaning software, drawing form and erected-in control mechanisms.
For automated systems, it’s anticipated that a cleaning instruction covers .
1) The claim cleaning phases, for illustration formerly- through versus re-circulating versus soak versus reflux-mode rinse/wash phases
2) The sequences of the cleaning phases
3) Frequency of the cleaning phases
4) Action applied during the cleaning process. record that the physical action/ impact is
frequently inflow/ pressure related (e.g. if spray balls are being used).
5) Used drawing agents and/ or drawing detergents
6) The attention and/ or quality of the used cleaning agents and/ or drawing detergents
7) Temperatures applied during the colorful cleaning phases.
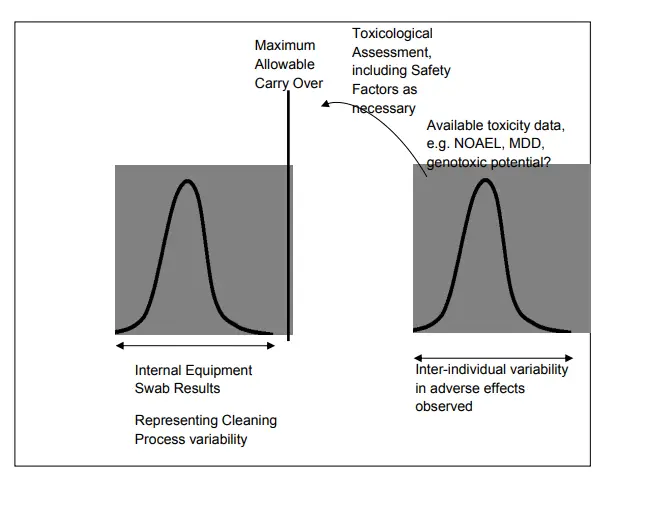
Due to the uncertainties on cleaning process parameters, like A.O. inflow, time, temperature, soap attention and starting conditions (inclusive Dirty Hold Time and smirching), and the geometric aspects of the gutted system, the cleaning process is susceptible to variability/ spread. The success of the cleaning process with its spread should be completely removed from the edge of failure of the cleaning process, which can be established by performing the MACO computations as bandied in the former chapters.
At lowest limit, cleaning should support a cleaning result below the attained MACO.
The position of cleaning should be commensurable to the position of threat that the cleaning process Acts in relation to the affiliated product processes. Notice that the cleaning threat can be further reduced either by
1) perfecting the cleaning cycle to ameliorate drawing effectiveness and shift the mean cleaning affect further down from the MACO position, which generally requires drawing development studies.
2) reducing process variability, which is generally established by adding the position of control on the cleaning process knowledge and may be used in a Process Analytical Technology (PAT) frame.
