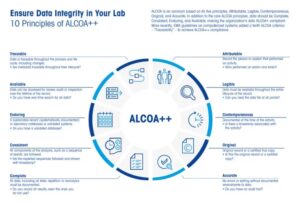
Alcoa++
In a world where data plays a crucial role in decision-making and technological advancements shape various industries, the significance of data integrity cannot be emphasized enough. Alcoa, a company renowned for its commitment to excellence, extends the principles of Alcoa Plus Plus beyond aluminum production to prioritize data integrity as a fundamental aspect of its operations. Let us explore how Alcoa++ principles seamlessly ensure robust data integrity within the organization.
The Foundation of Alcoa++: Accuracy
At the heart of Alcoa++ lies the principle of accuracy. Just as Alcoa maintains precise control over its aluminum manufacturing processes, the company places utmost importance on ensuring the accuracy of its data. Every piece of data generated within Alcoa undergoes rigorous checks to guarantee its accuracy, from production metrics to quality control measures.
Legibility and Accessibility: Key Aspects of Alcoa++
The commitment of Alcoa++ to legibility ensures that data is not only accurate but also presented in a clear and understandable format. This principle enhances accessibility, empowering decision-makers at all levels with the information they need to drive innovation and optimize processes. Transparent and legible data serves as a powerful tool that fuels informed decision-making across the entire organization.
Contemporaneous Recording: A Priority for Alcoa++
Both in manufacturing and data management, time is of the essence. Alcoa++ principles prioritize contemporaneous recording, ensuring that data is captured in real time. This not only enhances the organization’s responsiveness but also contributes to the reliability of historical records, enabling comprehensive analysis and trend identification.
Original Attributable, and Enduring Documentation:
Alcoa++ upholds its principles regarding documentation practices, with a focus on the importance of original, attributable, and enduring records. This guarantees that the data is not only genuine but also traceable to its source. By maintaining a comprehensive and well-documented data trail, Alcoa establishes a foundation for accountability and ongoing enhancement.
Consistency-A Pillar of Reliability:
Consistency serves as a fundamental principle within Alcoa Plus Plus, promoting standardized practices for data collection and reporting throughout the organization. This consistency ensures that the data is reliable and can be seamlessly integrated into various systems and processes. From the shop floor to the boardroom, consistent data forms the solid basis upon which strategic decisions are made.
Validation and Security:
The principles of Alcoa Plus Plus acknowledge the critical significance of data validation and security. Robust validation processes guarantee that the data is free from errors, while stringent security measures protect against unauthorized access and potential breaches. This dual commitment to validation and security underscores Alcoa’s dedication to preserving the integrity of its data assets.
In Conclusion:
As Alcoa++ extends its principles to encompass data integrity, it highlights the company’s comprehensive approach to excellence. By upholding accuracy, legibility, contemporaneous recording, and other fundamental principles, Alcoa ensures that its data is not merely a byproduct of its operations, but a strategic asset that drives innovation and efficiency. In a world where data integrity is of utmost importance, Alcoa Plus Plus sets a benchmark for organizations to seamlessly integrate these principles into their operations, fostering a culture of precision, transparency, and continuous improvement.
