Diphenhydramine HCL is Best cough medicine without dextromethorphan for treatment of sneezing, runny nose, watery eyes, hives, skin rash, itching, and other cold or allergy symptoms. Why Diphenhydramine HCL is used? Diphenhydramine is an antihistamine that reduces the effects of natural chemical histamine in the body. Histamine can produce symptoms …
Read More »Validation Questions on cleaning validation (APICS)
Validation Questions Question 1: When should a company validate/ revalidate cleaning procedures? When is validation not required? Answer: Ref. Section 7.0 and 10.0 Companies should look at each situation individually and determine the needfor validation. Section 7.0 provides a basic template, which may be used as a starting point in …
Read More »Cleaning process Control
Cleaning process Control To verify and qualify a cleaning process, the cleaning process needs to be unremarkable and sufficiently robust for the to-be-cleaned equipment. It would be clear which way are considered part of the product process /unit operation and which are part of the cleaning process, for Illustration if …
Read More »Operation, cleaning and maintenance of Static Pass Box.
Functioning, cleaning and maintenance of Static Pass Box. Objective: To lay down the method for the ordinary operation, cleansing and preservation of the Static Pass Box (SPB). Scope: This SOP is relevant to the Static Pass Box present inside the microbiology lab. Responsibility: Chemist or above of QC laboratory. Head …
Read More »Functioning, cleaning and maintenance of Dynamic Pass Box
Functioning, cleansing and maintenance of Dynamic Pass Box Objective: To lay down the method for the habitual operation, cleansing and renovation of the Dynamic Pass Box (DPB). Scope: This SOP is relevant to the Dynamic Pass Box present inside the microbiology lab Responsibility: Chemist or above of QC laboratory. Head …
Read More »Functioning, cleaning and maintenance of Sterile Garment Cabinet
Functioning, cleansing, and maintenance of Sterile Garment Cabinet Objective: To lay down the system for the ordinary operation, cleansing and protection of the Sterile Garment Cabinet (SGC). Scope: This SOP is relevant operation cleansing and protection of the Sterile Garment Cabinet present in the microbiology lab. Responsibility: Chemist or above …
Read More »SOP on Operation, Calibration, Cleaning and Maintenance of LAF
SOP on Functioning, Calibration, Cleaning, and Maintenance of LAF Objective: Technique for functioning, Calibration, Cleaning, and Maintenance of LAF. Scope: This SOP is relevant for the Operation, Calibration, Cleaning, and Maintenance of LAF in the Microbiology Laboratory. Responsibility: Chemist or above of Microbiology Laboratory. Head – Microbiology Section. Accountability: Head …
Read More »SOP on Maintenance and usage of Reference cultures
SOP on Maintenance and usage of Reference cultures Objective: SOP on Maintenance and usage of Reference cultures. Responsibility: Officer or above of Microbiology Laboratory: Accountability: Head – Microbiology section. Head – Quality control. Procedure Procurement of Lyophilized Cultures Reference Cultures can be procured from following suppliers: ATCC: American Type Culture …
Read More »Rubber Bung Processor Cum Sterilizer
Rubber Bung Processor Cum Sterilizer • HPHV Steam Sterilizer is used for sterilization of liquid, Hard and Hard Porous Material, Glass ware, drugs and surgical instrument used a pharmaceutical. It is also used For Sterilization of containers, vessels, linen, rubber articles culture media etc. • Provide with Sliding doors with …
Read More »Cleaning of Factory Footwear
Cleaning of Factory Footwear Objective To lay down a procedure for Cleaning of Factory Footwear. Scope This Standard Operating Procedure is applicable for cleaning of factory footwear in the formulation plant. Responsibility Housekeeping personnel is responsible to follow the procedure mentioned in SOP. HR Department is responsible for implementation of …
Read More »SOP on Document Management System in Quality Assurance Department.
Objective: To lay down a procedure for the Management of Documents in the Quality Assurance Department. Scope: Applicable for Document Management system in Quality Assurance department. Responsibility: Quality Assurance Department Accountability: Manager Q.A. is accountable for the compliance of this SOP. Procedure: Storage of Documents in Record Room. Documents shall …
Read More »Sterilization Process Controls
Sterilization Process Controls Inspectional Objectives Confirm that the sterilization process was validated by reviewing the validation study. Review the specific procedure(s) for the sterilization process selected and the methods for controlling and monitoring the process. Verify that the process is controlled and monitored. If review of the Device History Records …
Read More »Dry Powder Injection
Dry Powder Injection Majority of drugs show the problem of poor solubility, whether in the case of their analytical estimations or in the field of liquid dosage forms in the form of solutions. Commonly used organic solvents for spectrophotometric analysis of water insoluble drugs include methanol, ethanol, chloroform, benzene, dichloromethane, …
Read More »Key Point of Dry Powder Injection
The injectable dry filling area is a completely sterile area of the company that is a strictly controlled area. The high-level alertness is mandatory to main the atmospheric condition in the filling area of the dry powder filling area of Injectable. Every step in the production area requires a written …
Read More »DOCUMENTATION IN PHARMA
DOCUMENTATION IN PHARMA Well-established documentation system exists to implement Good Documentation Practices, which is a part of Quality Assurance. Clearly written documents are prepared, reviewed and controlled, which prevents the errors from verbal communication and permits traceability for all activities carried out. Quality Assurance is responsible for the revision and …
Read More »WATER SYSTEM
WATER SYSTEM: A purified Water system is a basic requirement for Pharmaceutical Industries. Raw Water sourced from industry bore well water is converted into purified water complying with current USP/ BP/ IP/ Ph. Eur. GENERATION OF PURIFIED WATER: The raw water passes through a sand filter through a transfer pump. …
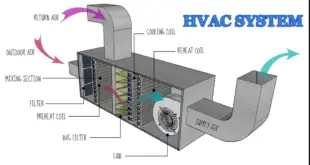
Read More »HEATING, VENTILATION AND AIR CONDITIONING (HVAC) SYSTEMS
BRIEF DESCRIPTION OF HEATING, VENTILATION, AND AIR CONDITIONING (HVAC) SYSTEMS: To avoid cross-contamination and to maintain relative humidity, temperature, and Pressure Difference of the manufacturing areas with Air handling Units, Air handling Units Provided in the manufacturing, warehouse, quality control, and microbiology section. Temperature and humidity are controlled and maintained …
Read More »BATCH MANUFACTURING RECORD OF DRY INJECTION
BATCH MANUFACTURING RECORD OF DRY INJECTION Table of content Batch Details General Instructions Calculation of API Dispensing of raw material API transfer record Dispensing of primary packing materials Component preparation for sterilization Steam sterilization record Vial de-boxing, Staging, and Inspection record Vial washing record Vial depyrogenation record API Canister weight …
Read More »WHO TRS1019 Appendix 3 – Cleaning validation Principle
WHO TRS1019 Appendix 3 – Cleaning validation Principle Content of Cleaning validation Principle 1.0 Principle 2.0 Scope 3.0 General 4.0 Cleaning validation protocols and reports 4.1 Cleaning validation protocols 4.2 Cleaning validation reports 8.0 Personnel 6.0 Equipment 7.0 Detergents 8.0 Microbiology 9.0 Sampling 9.1 General 9.2 Direct surface sampling (direct …
Read More »Quality Risk Management in Analytical Laboratory
Quality Risk Management in Analytical Laboratory The Quality of the Product Drugs or Drug Product is evaluated in Quality Control analytical laboratories, So It is important to understand that the “services” and “output” of a Quality Control / Analytical Laboratory is directly related to Safety, Identity, Strength, Purity (efficacy), Quality …
Read More »