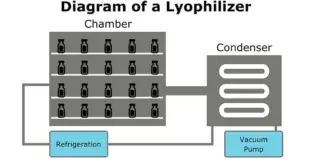
Lyophilization of Parenteral Lyophilization or freeze-drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase. The process consists of three separate, unique, and …
Read More »Equipment Qualification
Equipment Qualification Manufacturers must establish a systematic approach to qualification and validation to guarantee the quality, safety, and effectiveness of their products throughout their lifecycle. It is crucial to employ statistical evaluation when necessary to provide scientific evidence that the processes, systems, or other relevant aspects are appropriately qualified or …
Read More »Flow Properties of Powders
Flow Properties of Powders The use of powders in the pharmaceutical industry has generated a variety of methods for characterizing powder flow. Not surprisingly, scores of references appear in the pharmaceutical literature, attempting to correlate the various measures of powder flow to manufacturing properties. The development of such a variety …
Read More »IMPURITIES IN NEW DRUG SUBSTANCES
IMPURITIES IN NEW DRUG SUBSTANCES To provide guidance for registration applications on the content and qualification of impurities in new drug substances produced by chemical syntheses and not previously registered in a region or member state Impurities in new drug substances are addressed from two perspectives: Chemistry Aspects include classification …
Read More »Failure Mode Effect Analysis (Risk Assessment) Compression Stage
Failure Mode Effect Analysis (Risk Assessment) Compression Stage For More Pharma Updates Visit -https://pharmaguidances.com
Read More »Process validation scheme
Process validation scheme Traditional process validation Where validation data on production-scale batches are not provided with the application and traditional process validation is proposed, the process validation scheme described below should be submitted by the applicant. This should outline the formal process validation studies to be conducted on production-scale batches …
Read More »Process validation (Continuous process verification) for finished products
Process validation (Continuous process verification) for finished products Process validation can be defined as documented evidence that the process, operated within established parameters can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes (ICH Q7). Continuous process verification has been introduced to cover …
Read More »