Process validation scheme
Traditional process validation
Where validation data on production-scale batches are not provided with the application and traditional
process validation is proposed, the process validation scheme described below should be submitted by the applicant. This should outline the formal process validation studies to be conducted on production-scale batches (the number of batches used would depend on the variability of the process, the complexity of the process/product, and the experience of the manufacturer, but would usually be a minimum of 3 consecutive batches).
The information from these studies will be available for verification post-authorization by the supervisory authority. The process validation scheme should be submitted in the marketing authorization dossier and should include the
following information as a minimum:
- Short description of the process with a summary of the critical processing steps or critical process
parameters to be monitored during validation. - Finished product release specification (references to the dossier).
- Details of analytical methods (references to the dossier).
- In-process controls proposed with acceptance criteria.
- Additional testing is intended to be carried out (e.g. with proposed acceptance criteria and analytical
validation as appropriate). - Sampling plan – where, when, and how the samples are taken.
- Details of methods for recording and evaluation of results.
- Proposed timeframe.
Following completion of the scheme, a report containing the following information and signed by the
the appropriate authorized person should be generated and made available for inspection:
- Batch analytical data
- Certificates of analysis
- Batch production records.
- Report on unusual findings, modifications, or changes found necessary with appropriate rationale.
- Conclusions.
Where the results obtained show significant deviations from those expected, the regulatory authorities
need to be informed immediately.
In such cases, corrective actions should be proposed and any proposed changes to the manufacturing process should receive appropriate regulatory approval by way of variation.
Continuous process verification
In cases where continuous process verification is proposed a continuous process verification scheme should be submitted by the applicant. This should outline the monitoring to be performed on production-scale batches.
The information obtained will be available for verification post-authorization by the supervisory authority. The continuous process verification scheme should be submitted in the marketing authorization dossier and should include, as appropriate, the following information on the monitoring proposed:
- Details of online/in-line / at-line monitoring include parameters tested, number of samples, size of samples, and frequency of monitoring.
- Details of Analytical Methods (References to the dossier).
- Acceptance criteria.
- Information/ data including, as appropriate, information on statistical models or tools used to determine whether the continuous verification data support the ability of the process and controls to produce the reproducible product at a commercial scale.
- If a design space has been developed, how the proposed monitoring will contribute to design space
verification.
The presentations of solid oral dosage formulations are generally capsules, tablets, and powder /granules for solution/suspension. Solid oral dosage products could be packaged as unit dosage forms such as blisters and sachets or as multi-units in the form of bottles.
Capsules are solid dosage forms in which the drug is enclosed in a hard or soft soluble shell, commonly made of gelatine or starch, or other suitable substance. Capsules may be formulated for immediate or modified release of drugs that may be in the form of powder, liquids, or semisolids.
Capsules can also be filled with uncoated or coated pellets, mini-tablets, powder, or granules to permit transit through the stomach to the small intestine before the medication is released to alleviate potential problems of drug inactivation or gastric mucous irritation, as in the case of modified release dosage forms.
Tablets are solid dosage forms that contain medicinal substances with suitable excipients manufactured by direct compression of powders or granules with the application of high pressures, using steel punches and dies. Tablets can be of any size, weight, colour and shape, and may have surface markings.
Tablets can also be film-coated and/or have imprints Powder/granules for solution/suspension may be presented in single-dose units or multi-dose units and is required to be reconstituted in water before being administered orally.
Presentations in multidose units may be used where the strengths of each dose may not be critical.
Process validation of a solid oral dosage form has to be specific to its batch formula and the operating principles of equipment used for its manufacture. The process parameters that need to be controlled and/or monitored and testing that need to be conducted during process validation of bulk solid oral dosage formulations depend on its method of manufacture and its presentation (compressed tablet, coated tablet, capsule, powder/granule).
The acceptance criteria should take into consideration the nature of the solid oral dosage, for example its drug release characteristics (immediate-release (IR) or modified release (MR)). The following validation scheme can be used as a guide for process validation of solid oral dosage form and should be evaluated on a case-by-case basis.
VALIDATION SCHEME OF SOLID ORAL DOSAGE MANUFACTURING PROCESSES
The following items should be taken into account for the execution of process validation of the solid
oral dosage manufacturing process:
Batch Formula
For the execution of the manufacturing process validation, the batch formula of the solid oral dosage has to be well defined. All components of the dosage form to be used in the manufacturing process have to be listed, with their amounts on a per batch basis (including overages, if any).
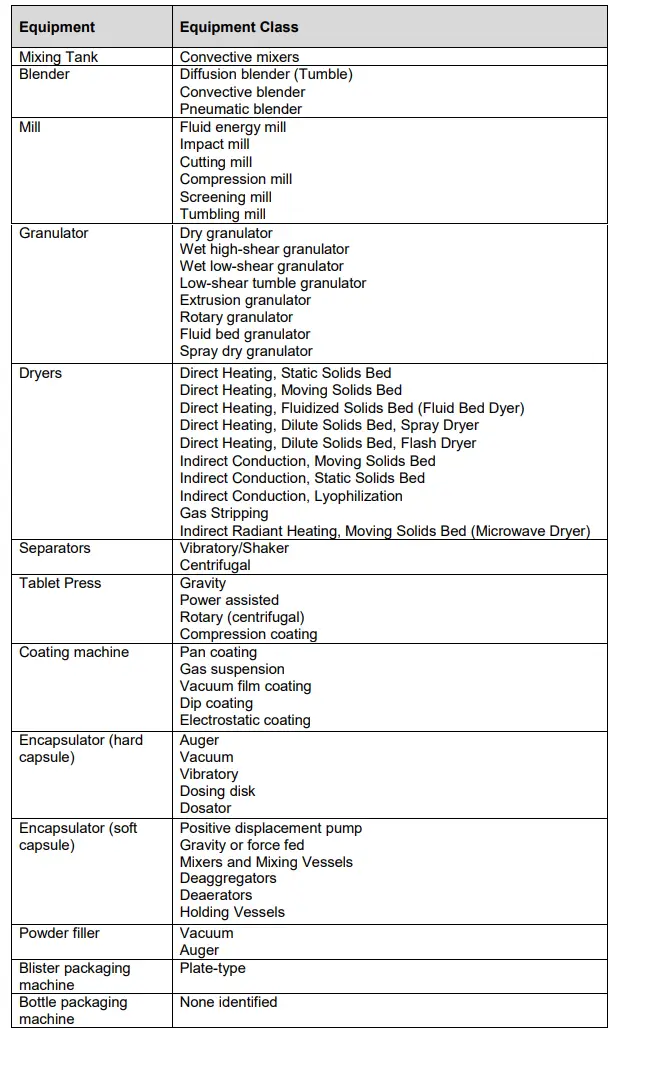
Major Equipment and Equipment Class
The major equipment, used for the manufacturing process, are to be identified and the class of each equipment be indicated. The equipment are broadly categorized by the unit operation (for example, blending and mixing, drying, particle size reduction, granulation, unit dosage, coating, encapsulation, printing, packaging). For each operation, the equipment is further categorized by class (operating principle).
The following lists some examples of equipment class for equipment of each major unit operation, which are non-exhaustive.
The product owner / applicant will determine the level of equipment information to be registered. Where information on the equipment class is deemed critical but not made available in the submission, the Drug Regulatory Authority (DRA) reserves the right to request for such information.
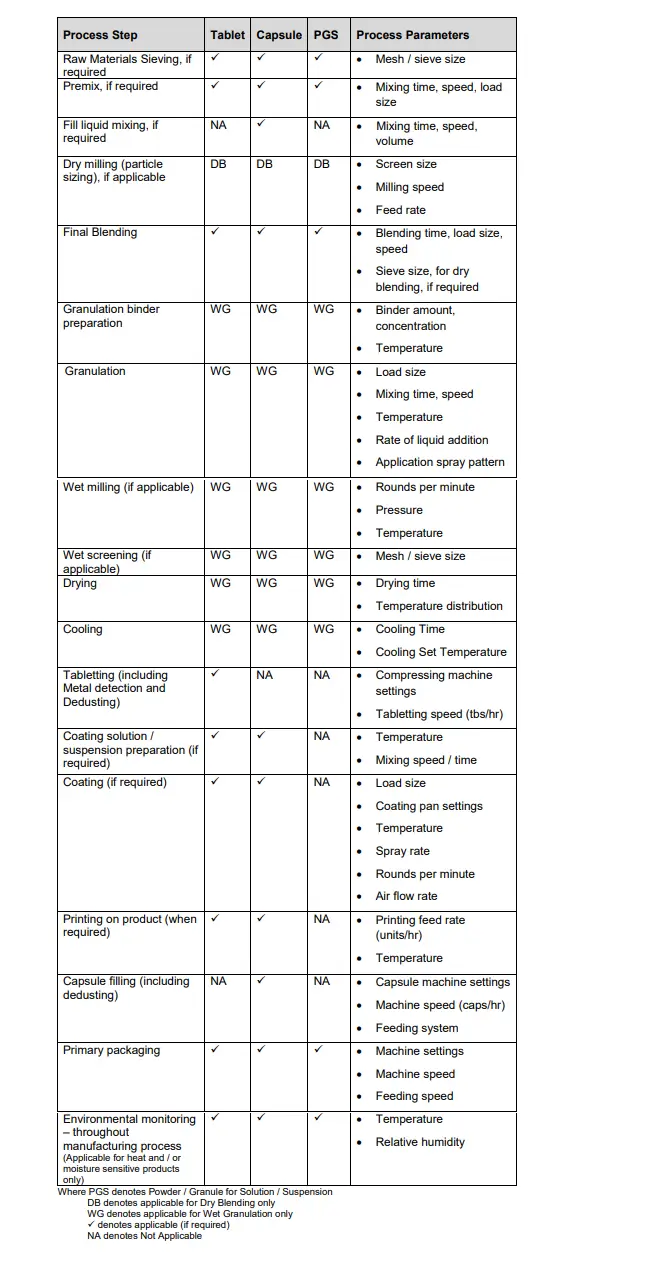
Manufacturing Process Description and Process Parameters
The manufacturing process may be described or presented in a flow diagram.
The following process parameters are recommended to be controlled or monitored as part of the process validation, depending on the dosage form and the type of manufacturing process.
The process parameters listed below are non-exhaustive. They serve only as examples and may differ depending on the class of equipment used.
The product owner/applicant will determine the level of process information to be registered.
Where process parameters are deemed critical but not well defined in the submission, the DRA reserves the right to request such information.
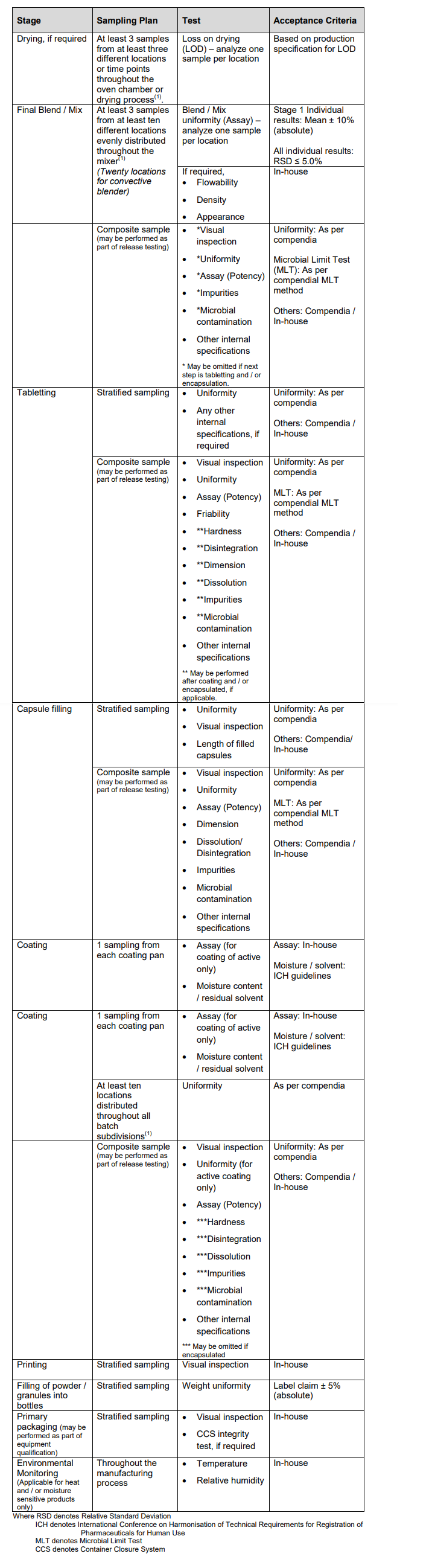
Sampling Plan and Acceptance Criteria
It is the responsibility of the manufacturer to ensure that the sampling plan and acceptance criteria defined are adequate to ascertain that the manufacturing process is well-controlled and robust to produce drug products consistently meeting specifications.
The following sampling plan and acceptance criteria provide a guide for the process validation of a typical solid oral dosage manufacturing process with a medium risk indication.
Note: Other sampling plans may be acceptable if they are statistically sound and justified.
The extent of sampling, tests and acceptance must take into consideration, the level of risk,e.g. the equipment type and capacity, to patient health of the drug product and should be considered on a case-by-case basis.
The finished product specifications have to be adequately justified and the analytical methods have to be validated as per the ASEAN Guidelines for Validation of Analytical Procedures.
Holding Time for Drug Products
Where holding times are involved as part of the manufacturing process of the bulk drug product (including the premix and intermediate stages), these have to be well justified. It is recommended for any holding times to be supported by stability data (degradation studies and / or microbial limit tests).
Holding time studies may be performed as part of the main process validation scheme or conducted as a separate exercise. Hold time may be established as a deliberate effort in that the samples or batches are withheld for the predetermined holding time before subjecting to analysis.
Holding time may also be established as part of the routine manufacturing process, using incurred holding times, which had been supported by data.
In the case where hold time information is not included in the submission, such information or justification/data to support the omission must be made available upon request of the DRA.
Reference: Guideline on process validation for finished products – information and data to be provided in regulatory
submissions