Stability Definition
- These studies provide information about the packaging in that it is not reactive, additive, or absorptive so that the identity, strength, quality and purity of the drug product is not affected, also to provide clearance on stability process flow.
- To assessment of the stability characteristics of all drug products manufactured / packaged and/or repacked by Pharmaceuticals companies.
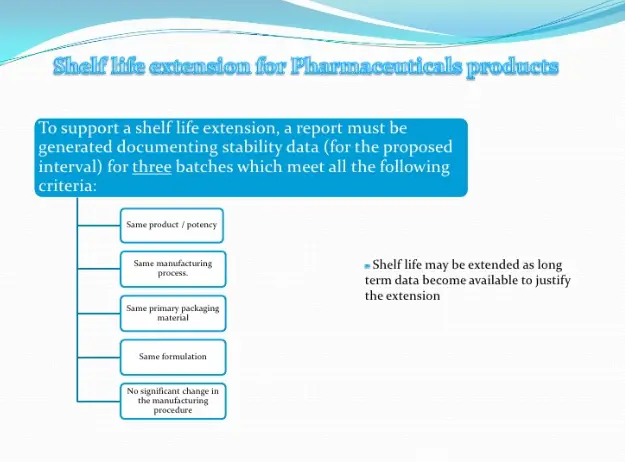
- To establish and extend the shelf life for products.
General Principles
The purpose of stability testing is to provide evidence on how the quality of an active substance or pharmaceutical product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light.
In addition, product-related factors influence the stability, e.g. the chemical and physical properties of the active substance and the pharmaceutical excipients, the dosage form and its composition, the manufacturing process, the nature of the container-closure system, and the properties of the packagingmaterials. Also, the stability of excipients that may contain or form reactive degradation products, have to be considered.
Active Substance
Information on the stability of the active substance is an integral part of the systematic approach to stability evaluation. For active substances not described in an official pharmacopoeial monograph, stability studies are required. For active substances described inan official pharmacopoeial monograph, which covers the degradation products and for which suitable limits have been set but a re-test period is not defined, two options are acceptable:
The manufacturer of the pharmaceutical product confirms that the active substance complies with the pharmacopoeial monograph immediately prior to the manufacture of the pharmaceutical product. In this case no stability studies on the active substance are required. The suitability of the pharmacopoeial monograph for the active substance used from a named source of supply has to be demonstrated.
Stress Testing
Stress testing of the active substance can help identify the likely degradation products, whichcan in turn help establish the degradation pathways and the intrinsic stability of the moleculeand validate the stability indicating power of the analytical procedures used. The nature of thestress testing will depend on the individual active substance and the type of pharmaceuticalproduct involved.
Stress Testing for an active substance the following approaches may be used:
a) When an active substance is described in an official pharmacopoeial monograph, andfully meets its requirements, no data are required on the degradation products if theyare named under the headings “purity tests” and/or “section on impurities
b) For active substances not described in an official pharmacopoeial monograph, there aretwo options:− When available, it is acceptable to provide the relevant data published in theliterature to support the proposed degradation pathways;− When no data are available in the scientific literature, including officialpharmacopoeias, stress testing should be performed.
Specification
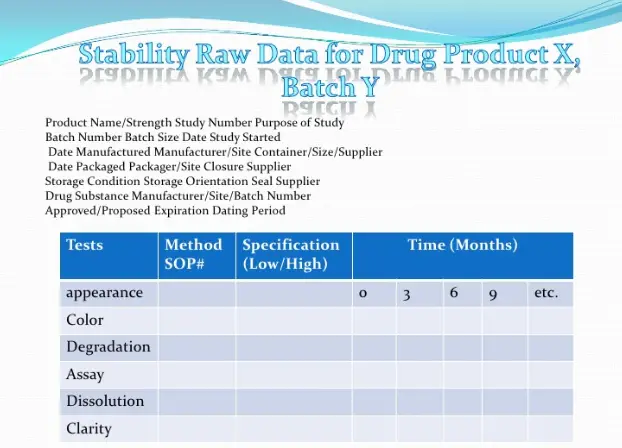
Stability studies should include testing of those attributes of the active substance that aresusceptible to change during storage and are likely to influence quality, safety, and/or efficacy.The testing should cover, as appropriate, the physical, chemical, biological, andmicrobiological attributes. Validated stability-indicating analytical procedures should beapplied. Whether and to what extent replication should be performed will depend on the resultsfrom validation studies
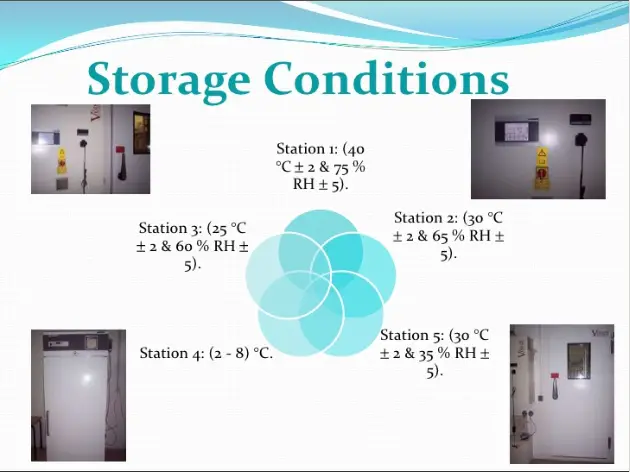
Storage Conditions
In general, an active substance should be evaluated under storage conditions (with appropriatetolerances) that test its thermal stability and, if applicable, its sensitivity to moisture. Thestorage conditions and the lengths of studies chosen should be sufficient to cover storage,shipment, and subsequent use with due regard to the climatic zone(s) in which the activesubstance is intended to be stored .
Testing Frequency
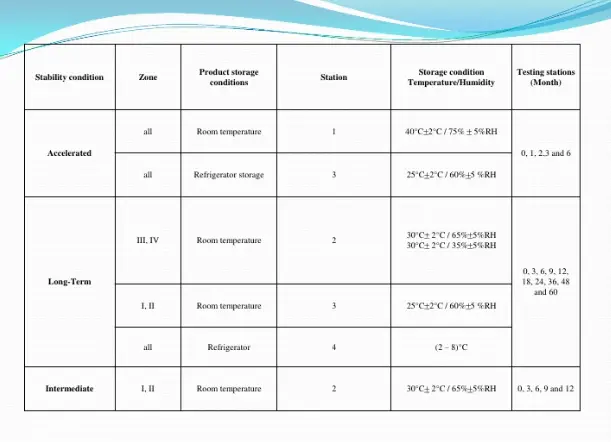
For long term studies, frequency of testing should be sufficient to establish the stability profile of the active substance. For active substances with a proposed re-test period of at least 12 months, the frequency of testing at the long term storage condition should normally be every three months over the first year, every six months over the second year, and annually thereafter through the proposed re-test period.
At the accelerated storage condition, a minimum of three time points, including the initial and final time points (e.g. 0, 3, and 6 months), from a 6- month study is recommended. Where an expectation (based on development experience) exists that results from accelerated studies are likely to approach significant change criteria, increased testing should be conducted either by adding samples at the final time point or by including a fourth time point in the study design
Type of Placement Batches:
A- New product registration.
B- Process Validation Batch.
C- Formulation Change.
D- Annual Commitment Batch.
E- Raw material manufacturer change
F- Primary packaging material manufacturer change
G- Reprocessing batch
H- Batch size change
I- Manufacturing process change
J- Stability condition change
K- Machine change
L- Site transfer
In-use stability
The purpose of in-use stability testing is to establish – where applicable – the period of timeduring which a multi-dose product can be used whilst retaining acceptable quality once thecontainer is opened and the first dose is removed.A minimum of two batches, at least pilot scale batches, should be subjected to the test. At leastone of the batches should be chosen towards the end of its shelf life. If such results are notavailable, one batch should be tested at the final point of the submitted stability studies.
Variations
Once the pharmaceutical product has been registered, additional stability studies are requiredwhenever major variations are made like the following:1. Change in the manufacturing process;2. Change in the composition of the pharmaceutical product;3. Change of the immediate packaging.
Stability tests for dosage forms:
1. Tablets
Dissolution (or disintegration, if justified), water content and hardness/friability. For coated and colour tablets additional tests may require for texture and colour stability.
2. CapsulesHard gelatin capsules: brittleness, dissolution (or disintegration, if justified), water content, and level of microbial contamination.
3. EmulsionsPhase separation, pH, viscosity, level of microbial contamination, and mean size and distribution of dispersed globules.
4. Oral solutions and suspensionsFormation of precipitate, clarity for solutions, pH, viscosity, extractables, level of microbial contamination.
Additionally for suspensions, redispersibility, rheological properties, mean size anddistribution of particles should be considered. Also, polymorphic conversion may be examined, if applicable.
5. Powders and granules for oral solution or suspensionWater content, and reconstitution time.Reconstituted products (solutions and suspensions) should be evaluated as described in“Oral solutions and suspensions” above, after preparation according to the recommendedlabeling, through the maximum intended use period.
6. Nasal sprays: solutions and suspensionsClarity (for solution), level of microbial contamination, pH, particulate matter, unit spraymedication content uniformity, number of actuations meeting unit spray content uniformityper container, droplet and/or particle size distribution, weight loss, pump delivery,microscopic evaluation (for suspensions), foreign particulate matter andextractable/leachable from plastic and elastomeric components of the container, closure andpump.
7. Topical, ophthalmic and otic preparationsIncluded in this broad category are ointments, creams, lotions, paste, gel, solutions, eyedrops, and cutaneous sprays.
Topical preparations should be evaluated for clarity, homogeneity, pH, resuspendability (forlotions), consistency, viscosity, particle size distribution (for suspensions, when feasible), level of microbial contamination/sterility and weight loss (when appropriate).Evaluation of ophthalmic or otic products (e.g. creams, ointments, solutions andsuspensions) should include the following additional attributes: sterility, particulate matter and extractable. Evaluation of cutaneous sprays should include: pressure, weight loss, net weight dispensed, delivery rate, level of microbial contamination, spray pattern, water content, and particle size distribution (for suspensions).
8. SuppositoriesSoftening range, dissolution (at 37°C).
9. Small volume parenterals (SVPs)Colour, clarity (for solutions), particulate matter, pH, sterility, endotoxins.Stability studies for powders for injection solution should include monitoring for colour, reconstitution time and water content. Specific parameters to be examined at appropriate intervals throughout the maximum intended use period of the reconstituted drug product, stored under condition(s) recommended in labelling, should include clarity, colour, pH, sterility, pyrogen/endotoxin and particulate matter.The stability studies for Suspension for injection should include, in addition, particle sizedistribution, redispersibility and rheological properties.The stability studies for Emulsion for injection should include, in addition, phase separation,viscosity, mean size and distribution of dispersed phase globules.
10. Large volume parenterals (LVPs)
Colour, clarity, particulate matter, pH, sterility, pyrogen/endotoxin, and volume.
When re-test is done??
In general, “significant change” for a pharmaceutical product is defined as:1. A 5% change in assay from its initial value; or failure to meet the acceptance criteriafor potency when using biological or immunological procedures;2. Any degradation product exceeding its acceptance criterion;3. Failure to meet the acceptance criteria for appearance, physical attributes, andfunctionality test (e.g., colour, phase separation, resuspendibility, caking, hardness,dose delivery per actuation); however, some changes in physical attributes (e.g.,softening of suppositories, melting of creams, partial loss of adhesion for transdermal products
4. Failure to meet the acceptance criterion for pH; or
5. Failure to meet the acceptance criteria for dissolution for 12 dosage units.
Ongoing stability studies
The stability of the API should be monitored according to a continuous and appropriate program that will permit the detection of any stability issue (e.g. changes in levels of degradation products). The purpose of the ongoing stability program is to monitor the API and to determine that the API remains, and can be expected to remain, within specifications under the storage conditions indicated on the label, within the re-test period in all future batches.The ongoing stability program should be described in a written protocoland the results presented in a formal report.
After registration…
Once the pharmaceutical product has been registered, additional stability studies are requiredwhenever variations that may affect the stability of the active pharmaceutical substance orpharmaceutical product are made, such as major variations like the following:a. Change in the manufacturing process.b. Change in the composition of the pharmaceutical product.c. Change of the immediate packaging.
At the market…
If no significant change occurs during six-months accelerated and real time stability testing,the product will be allowed to place in the market with a provisional shelf-life of up totwenty-four months. However, real time stability testing should be continued up to theproposed shelf-life. The manufacturer should have a system of recall in place so that the saleany batch which does not remain within the limit of approved product specification bestopped within twenty-four hours.