DISPENSING & SAMPLING BOOTH
The sampling Booth or Dispensing Booth is designed to provide high levels of protection from airborne contaminants generated during powder handling operations such as charging & dispensing. ( Sampling Booth or Dispensing Booth ) provides Operator Protection, Products Protection, and Environment Protection.
The Sampling Booth or Dispensing Booth operates on a re-circulatory airflow principle providing containment by air movement. A clean, constant, laminar down flow of Class 100 air is supplied in the work area suppressing any dust clouds generated during open powder processing: removing and capturing airborne particles from the operator’s breathing zone.
Salient Features of Dispensing Booth
- Material of Construction: SS 304 / SS 316 / SS 316 L
- Triple Stage Filtration
- EU4-Prefilters (90% down to 5 micron)
- EU7-Intermediate Filter (95% down to 3 micron)
- EU13- HEPA Filter-Supply / Bleed (99.997% down to 0.3 micron)
- LED Lights & Differential Pressure Gauge for all 3 Filters
- ON/OFF Switch
- PAO Test Port
- 5/15 Amp Switch Socket for external equipment
OPERATING CONDITIONS
- Air cleanliness: ISO Class 5 (ISO 14644-1:1999 (E))
- Air Velocities: 0.45+_ 0.05mps
- Air Flow: Vertical – Re-circulatory
- Noise Level: Less than 67 dB
- Vibration Level: Minimum
- Minimum: > 300 lux
- Power Supply: 230V AC 1-0 50 HZ
TECHNICAL SPECIFICATIONS:
PRE‐FILTER (PRIMARY FILTER): –
- Filter Casing‐ SS 304/316 flange types.
- Efficiency‐ 90% down to 5 microns (EU‐4 Ratings.)
- High-Density Polyethylene With Three Layers
INTERMEDIATE‐FILTER (SECONDARY FILTER): –
- Filter Casing‐ AL flange type.
- Efficiency‐ 95% down to 3 microns (EU‐7 Rating.)
- Sandwiched Between Two Layers of HDPE Mesh Non‐woven Synthetic Media.
BLOWER MOTORS ASSEMBLY: –
- Electric Motors double-ended shaft, Single phase, 240 volt, 50Hz.
- Impellers‐Aluminum, static & dynamic balanced, forward curve types.
- Blower casing‐ AL / GI / MS, centrifugal type with spring suspension.
ACCESSORIES:
- 100 % Exhaust System
- Downflow Booth with Biological Safety Application
- Booth Compatible for Solvent use
- Pass box Integration
- Double skin solid side panels with/without view windows
- Stainless steel perforated grille for supply HEPA Filters
- Front Anti-Static PVC Curtains
- Sodium Vapor Lamps
- Night Mode Operation
- Velocity display with alarms (audible and visible)
- Filter blocked alarms (audible and visible)
- Explosion-proof electrics for Flameproof applications
- PLC-based Control System
- Once-through airflow systems
- Safe change Bag in/Bag out arrangement for filters
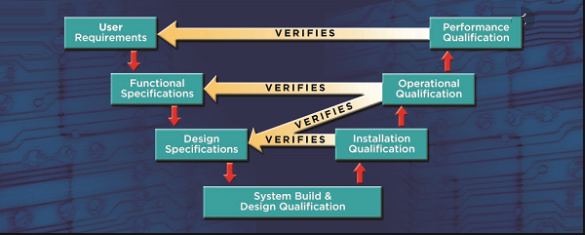
INSTALLATION QUALIFICATION PROTOCOL OF DISPENSING BOOTH
TABLE OF CONTENT
- Purpose
- Scope
- Responsibilities
- Procedure
- Installation Qualification tests
- Documents & Drawings Verification
- Verification of Technical Specifications for In-House & sub-components / Bought out items
- Utility Verification
- Material of Construction Verification
- Critical Instrument Calibration Verification
- Installation Qualification Tests Status
- Data Analysis, Summary of IQ & Recommendations
- Amendment Record
- Conclusion
PURPOSE
To describe the Installation Qualification of Open Fronted Containment Facility, and its accessories and to define the Specification of the system to:
- Aid verification of the installation as per equipment general arrangement drawing.
- Ensure that the system installation meets acceptance criteria.
- Ensure that the equipment will be installed by current Good Manufacturing Practices.
SCOPE:
The scope of this document applies to the installation qualification of Open Fronted Containment Facility and its accessories to be installed at a Pharmaceutical Company.
RESPONSIBILITIES:
It is the responsibility of the Validation Team to prepare and check installation qualification protocol.
It is the responsibility of the Quality Assurance approve the installation qualification protocol.
PROCEDURE
The following requirements/practices apply to Open Fronted Containment Facility, Installation Qualification activities:
Verify that systems are installed in accordance
-
- General arrangement drawing.
- Installation specification; and
Verify that the equipment and instrumentation are clearly described as per vendor, model, capacity, materials of construction, and any critical criteria.
Verify that major components are tagged or labeled with a unique ID number.
INSTALLATION QUALIFICATION TESTS
The table below lists the tests to be performed as part of the Installation Qualification phase.
CRITICAL FEATURE
- Documents & Drawings Verification
- Verification of Technical Specifications for In-House & sub-components / bought-out items
- Utility Verification
- Material of construction verification
- Critical instrument Calibration Verification
Documents & Drawings Verification
Rationale –
To verify that the documentation provides complete and correct technical references and permits servicing of the units.
Test equipment –
Not Required.
Procedure –
-
- Verify that the required documents and drawings listed in the table are available.
- Review the documents and drawings for completeness and exactness with the installed units.
- Attach the copies of the drawings to this document or reference the location from where they can be easily retrieved.
- Any items in the installation, which are not conform to the corresponding drawings, must be commented on in the appropriate space. List the non-conformances and the reasons for them in an attachment if necessary.
Acceptance Criteria –
-
- The documents must be accurate and complete.
- The drawing information must correspond to the physical installation.
- Drawing Verification Results:
| Sr. No | Description | Document No | Verified By |
| 1 | General arrangement drawing |
Verification of Technical Specifications for In-House & sub-components / Bought out items
Purpose
To verify that each major component of the Klenzaids Product, Open Fronted Containment Facility, is present and identified.
Test Equipment
None Required
Procedure
Confirm that identification nameplates have been applied to the units and that these indicate the following (where applicable).
– Type
– Identification (Tag)
– Manufacturer
– Model Number
Confirm that the various components of Klenzaids Product, Open Fronted Containment Facility, are present and tagged as per specifications and drawings, in the proper number and configuration.
Note any deviations or discrepancies and recommend follow-up action if required.
Acceptance Criteria
All units and parts specified in drawings must be present, documented, and tagged.
Major Components Results
| Equipment Description | |
| Description | Specification |
| Name | Open Fronted Containment Facility Model: OFCF-32 |
| Overall dimensions | 1870 mm (W) x 2800 mm (L) x 2480 mm (H) |
| HEPA Filter (Exhaust) | |
| Micron Rating | 0.3µ |
| Filter Efficiency | 99.97% of 0.3µ |
| Size |
1205 mm x170mm x75mm |
| Filter media |
Fiber Glass |
| Make |
Klenzaids Bioclean Devices (P) Ltd. |
| HEPA Filter | |
| Micron Rating | 0.3µ |
| Filter Efficiency | 99.97% of 0.3µ |
| Size | 1220 mm x 610mm x75mm |
| Filter media |
Fiber Glass |
| Make |
Klenzaids Bioclean Devices (P) Ltd. |
| HEPA Filter | |
| Micron Rating | 0.3µ |
| Filter Efficiency | 99.97% of 0.3µ |
| Size | 1220 mm x 640mm x75mm |
| Filter media | Fiber Glass |
| Make | Klenzaids Bioclean Devices (P) Ltd. |
| 5. Intermediate Filter | |||||
| Sr. No. | |||||
| Micron Rating | 3µ | ||||
| Size | 1140 mm x 420 mm x 45 mm | ||||
| Flow Rate | 750 cfm at 0.20” | ||||
| Filter media | Fiber Glass Media | ||||
| MOC of frame | S.S. | ||||
| Gasket | Neoprene | ||||
| Qty. | 02 Nos. | ||||
| 6. Prefilter | |||||
| Sr. No. | |||||
| Micron Rating | 5µ | ||||
| Size | 910 mm x 600 mm x 45mm | ||||
| Flow Rate | 625 cfm at 0.15” | ||||
| Filter media | Fiber Glass Media | ||||
| MOC of frame | S.S. | ||||
| Gasket | Neoprene | ||||
| Qty. | 04 Nos. | ||||
| 7. Motor Blower | |||||
| Sr. No. | |||||
| Motor Construction | Non Flame Proof | ||||
| Power Rating | 1.0 HP / 3 ph / 415 v / 1.8 amp | ||||
| Motor RPM | 1440 rpm | ||||
| Make | Klenzaids Bioclean Devices (P) Ltd. | ||||
| Qty. | 02 Nos. | ||||
| 8. Magnehelic Pressure Gauge | |||||
| Range | 0-25 mm WC | ||||
| Leas count | 0.5 mm WC | ||||
| Make | Dwyer | ||||
Utility Verification
Rationale –
To verify that all necessary utilities are correctly installed.
Procedure –
-
- Confirm that utility connections are configured as per specification and in compliance with local codes.
- Record the results in the table below. Note any deviations or discrepancies.
Acceptance Criteria
-
- All services and connections must be installed and documented.
Utility Specification Results
| Description | Specified | Observation |
| Electrical | To be provided |
Material of Construction Verification
Rationale –
To verify that all assembly of Klenzaids Product, Open Fronted Containment Facility has been manufactured as per the specification provided by the customer.
Test equipment –
Moly testing unit (To identify 304 or 316 materials)
Procedure –
-
- Put a drop of Molybdenum solution on the material to be tested.
- Take battery & keep anode at one end of the material and cathode at Moly drop.
- If the solution turns pink and stays for around one minute, then it is SS 316 & if the solution turns pink and immediately vanishes, then it is SS 304
Acceptance Criteria
-
- All material of construction for the Open Fronted Containment Facility shall meet SS 304 std.
- Material other than SS316 and SS304 are verified based on their material testing certificates provided by the Manufacturer.
Material of Construction Verification Results
| Description | Specification | Meets Spec. (Yes/No) | Verification Source |
| Body of equipment | S.S. 304 [18 SWG] |
Critical Instrument Calibration Verification
Rationale-
To verify that all critical instruments for the Open Fronted Containment Facility have been calibrated before starting the Operational Qualification.
Procedure-
-
- Verify that all critical instruments are calibrated using an approved procedure, against a traceable standard.
- Note any deviations or discrepancies and recommend follow-up actions if required.
- For all critical instruments, attach copies of the calibration certificate to this protocol.
Acceptance Criteria
All critical instrumentation for Open Fronted Containment Facilities shall be in a state of calibration.
Critical Instrument Calibration Verification
| Description | Manufacturer | Current Calibration | ||
| Yes/No | Sign / Date | |||
| Megnehelic Gauge | ||||
Installation Qualification Tests Status
The table below lists the tests performed and related results.
| Test Number
|
Critical Feature
|
Pass / Fail | Deviation Found | ||
| Pass | Fail | Yes | No | ||
| Documents & Drawings Verification | |||||
| Verification of Technical Specifications for In-House & Sub-contract / bought-out items. | |||||
| Utility Verification | |||||
| Material of Construction Verification | |||||
| Critical Instrument Calibration Verification | |||||
Data ANALYSIS, Summary of IQ & Recommendations
AMENDMENT RECORD
Note: Any changes made in the system must be recorded in this sheet.
CONCLUSION