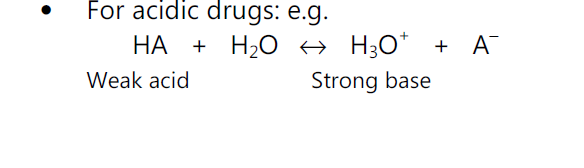Characterization of Fine Particle in Formulation of drugs
Parameters those are measured:
(i) Particle size and size-distribution
(ii) Shape of the particle
(iii) Surface morphology of the particles
(iii) Zeta potential
Instrumental Methods of Particle Size Characterization:
(i) Light Microscope:
• First a standard graticule (BS 3625) is standardized with a stage micrometer. Then small number of particles are spread over a glass slide and placed on the stage of the microscope. Particles are focussed and the particle diameters are measured.
Several hundred particles are measured and reported as a histogram.
• Disadvantage: The procedure is tedious (means it takes slow and monotonous long time.)
(ii) Stream counting devices:
Examples:
(a) Coulter counter – electrical sensing zone method
(b) HIAC – counter – optical sensing zone
(c) Malvern particle and droplet sizer – Laser diffraction method.
Procedure: Vacuum Amplifier and Counter Stirrer Electrodes Orifice:
• Samples prepared for analysis are dispersed in a conducting medium (e.g. saline) with the help of ultrasound and a few drops of surfactant (to disperse the particles uniformly). A known volume (0.5 to 2 mL) of this suspension is then drawn into a tube through a small aperture (0.4 to 800 μm diameter) across which a voltage is applied.
• As each particle passes through the hole, it is counted and sized according to the resistance generated by displacing that particle’s volume of conducting medium.
• Size distribution is reported as histogram.
(iii) Sieve analysis:
• A powder sample is passed through a standard sieve set. The particle size is plotted against % weight retained on each sieve.
• Use: This method is used generally for large samples.
Instrumental Method for Determination of Specific Surface Area:
Brunauer, Emmett and Teller (BET) Nitrogen Adsorption Method:
• A layer of nitrogen molecules is adsorbed to the sample surface at –1960oC. Once the surface is drenched, the sample is heated to room temperature, the nitrogen gas is desorbed, and its volume is measured and converted to the number of adsorbed molecules via the gas law. Since each N2 molecule occupies an area of 16 A2, one may readily compute the surface area per gram of each pre-weighed sample.
Instrumental method for characterization of surface morphology:
• The scanning electron microscope creates the magnified images by using electrons instead of light waves. The images are black and white.
Procedure:
Biological materials are dried in a special way that prevents them from shrinking.
• Since SEM illuminates them with electrons, they are made conductive by coating with a very thin layer of gold by a machine called sputter-coater.
• The sample is placed inside the microscope’s vacuum column through an airtight
door.
• After the air is pumped out of the column, an electron gun emits a beam of high energy electrons. This beam travels downward through a series of magnetic lenses designed to focus the electrons to a very fine spot.
• Near the bottom, a set of scanning coils moves the focused beam back and forth across the specimen, row by row.
• As the electron beam hits each spot on the sample, secondary electrons are knocked loose from its surface. A detector counts these electrons and sends the signals to an amplifier.
• The final image is built up from the number of electrons emitted from each spot on the sample.
Bulk Density:
Apparent bulk density (g/cm3): Bulk drug powder is sieved through 40 mesh screen.
Weight is taken and poured into a graduated cylinder via a large funnel. The volume is called bulk volume.
Apparent bulk density = Weight of the powder / Bulk volume
Tapped density (g/cm3):
Bulk powder is sieved through 40 mesh screen. Weight is taken and poured into a graduated cylinder. The cylinder is tapped 1000 times on a mechanical tapper apparatus. The volume reaches a minimum – called tapped volume.
Tapped density = Weight of the powder / Tapped volume
True density (g/cm3):
Solvents of varying densities are selected in which the powder sample is insoluble. Small quantity of surfactant may be mixed with the solvent mixture to enhance wetting and pore penetration. After vigorous agitation, the samples are centrifuged briefly and then left to stand undisturbed until floatation or settling has reached equilibrium.
The samples that remains suspended (i.e. neither suspended not floated) is taken. So the true density of the powder is equal. So the true density of the powder is the density of that solvent. The density of that solvent is determined accurately with a pycnometer.
Source of Variation of Bulk Density:
Method of crystallization, milling, formulation
Methods of correction:
By milling, slugging or formulation
Significance:
Bulk density: Bulk density is required during the selection of capsule size for a high dose drug.
• In case of low dose drug mixing with excipients is a problem if the bulk densities of the drug and excipients have large difference.
• Near the bottom, a set of scanning coils moves the focused beam back and forth across the specimen, row by row.
• As the electron beam hits each spot on the sample, secondary electrons are knocked loose from its surface. A detector counts these electrons and sends the signals to an amplifier.
• The final image is built up from the number of electrons emitted from each spot on the sample.
4. Powder flow properties:
Powder flow properties depend on
(i) particle size
(ii) density
(iii) shape
(iv) electrostatic charge and adsorbed moisture
that may arise from processing or formulation.
A free-flowing powder may become cohesive during development. This problem may be solved by any of the following ways:
(i) by granulation
(ii) by densification via slugging
(iii) by filling special auger feed equipment (in case of powder)
(iv) by changing the formulation.
Procedure:
For free flowing powder: A simple flow rate apparatus consists of a grounded metal tube from which drug flows through an orifice onto an electronic balance, which is connected to a strip chart recorder. Several flow rate (g/sec) determinations at various orifice sizes (1/8 to ½ inch) should be carried out.
The greater the standard deviation between multiple flow rate measurements, the greater will be the weight variation of the product (tablets or capsules).
Compressibility:
Compressibility = rt − r0 / rt × 100
where rt = Tapped bulk density
r0 = Initial bulk density
Solubility Data
Determination of equilibrium solubility of a drug:
The drug is dispersed in a solvent. The suspension is agitated at a steady temperature.
Samples of the suspension are withdrawn as a function of time, clarified by centrifugation, and assayed to establish a plateau concentration.
Solvents taken:
(i) 0.9% NaCl at room temperature
(ii) 0.01 M HCl at RT
(iii) 0.1 M HCl at RT
(iv) 0.1 M NaOH at RT
(v) At pH 7.4 buffer at 37oC
Drug concentration is determined by the following analytical methods:
(i) HPLC
(ii) UV –Spectroscopy
(iii) Fluorescence Spectroscopy
(iv) Gas Chromatography
Solubility depends on
(i) pH
(ii) Temperature
(iii) Ionic strength
(iv) Buffer concentration
Significance:
(i) A drug for oral administrative should be examined for solubility in an isotonic saline solution and acidic pH. This solubility data may provide the dissolution profile invivo.
(ii) Solubility in various mediums is useful in developing suspension or solution toxicologic and pharmacologic studies.
(iii) Solubility studies identify those drugs with a potential for bioavailability problems.
E.g. Drug having limited solubility (7 %) in the fluids of GIT often exhibit poor or
erratic absorption unless dosage forms are tailored for the drug.
pKa Determination:
• When a weakly acidic or basic drug partially ionizes in GI fluid, generally, the unionized molecules are absorbed quickly.
• Handerson-Hasselbach equation provides an estimate of the ionized and unionized drug concentration at a particular pH.
Methods of Determination of pKa of a Drug:
(i) Detection of spectral change by UV or visible spectroscopy at a range of pH:
Advantage: Dilute aqueous solutions can be analyzed by this method.
(ii) Potentiometric titration:
Advantage: Maximum sensitivity for compounds with pKa in the range of 3 to 10.
Disadvantage: This method is unsuccessful for candidates where precipitation of the unionized forms occurs during titration. To prevent precipitation a co-solvent e.g. methanol or dimethyl sulfoxide (DMSO) can be incorporated.
• Difference of solubility at various pH.
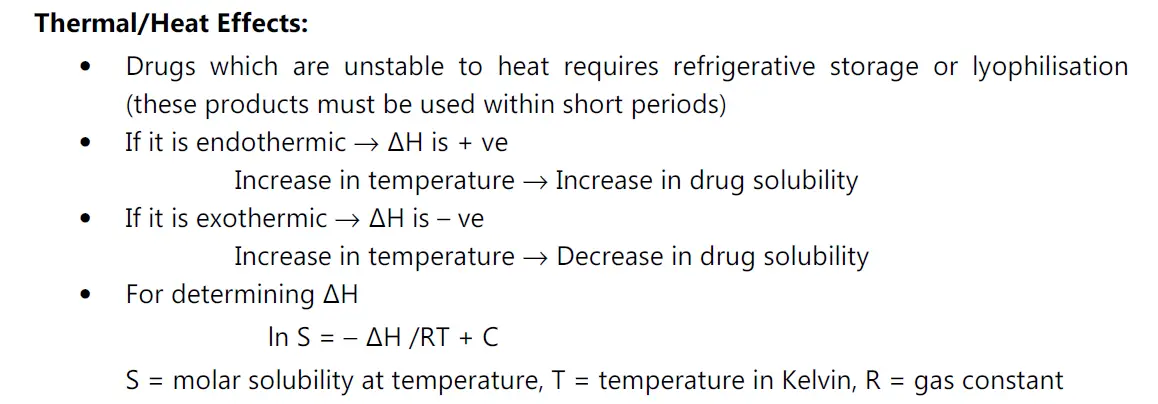
(iii) Effect of temperature on stability:
• Heat of solution, ΔHS represents the heat released or absorbed when a mole of solute is dissolved in a large quantity of solvent.
Significance:
• Most normally, the solubility process is endothermic, e.g. non-electrolytes, unionized forms of weak acids and bases. if ΔHs is positive, solubility increases if temperature increases.
• Solutes that are ionized when dissolved releases heat, the process is exothermic, ΔHs is negative solubility increases as temperature decreases.
(iv) Solubilization:
• For drug candidates with poor water solubility, preformulation studies should include limited experiments to identify the possible mechanisms for solubilisation.
• Means of increasing the solubility are:
(i) Addition of a cosolvent to the aqueous system e.g. ethanol, propylene glycol and glycerine.
MOA: These co-solvents disrupt the hydrophobic interactions of water at the non-polar solute / water interfaces.
(ii) Solubilisation in micellar solutions such as 0.01 M Tween 20 solution.
(iii) Solubilisation by forming molecular complexes e.g. benzoic acid forms complex with caffeine.
(v) Partition coefficient:
• Partition coefficient is defined, as the ratio of un-ionized drug concentrations between the organic and aqueous phases, at equilibrium.
• Generally, 2-octanol and chloroform are taken as the oil phase. m equilibrium Significance: Drug molecules having higher KO/W will cross the lipid cell membrane.
(vi) Dissolution:
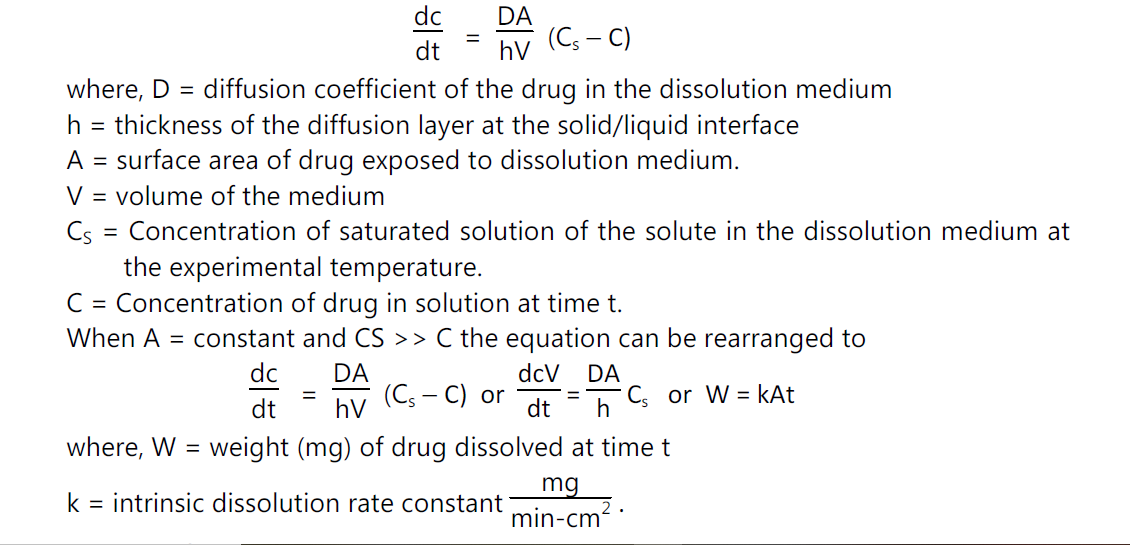
• The dissolution rate of a drug substance in which surface area is constant during disintegration is described by the modified Noyes-Whitney equation
Determination of k:
• Pure drug powder is punched in a die and punch apparatus to give a uniform cylindrical shape. The tablet is covered with wax in all sides. One circular face is exposed to the dissolution medium. Thus, as dissolution precedes, the area, A, remains constant.
• Time to time dissolution medium is taken out and fresh medium added to the chamber.
• With two types of assembly, the experiments can be carried out.
pH and pKa Solubility Profile:
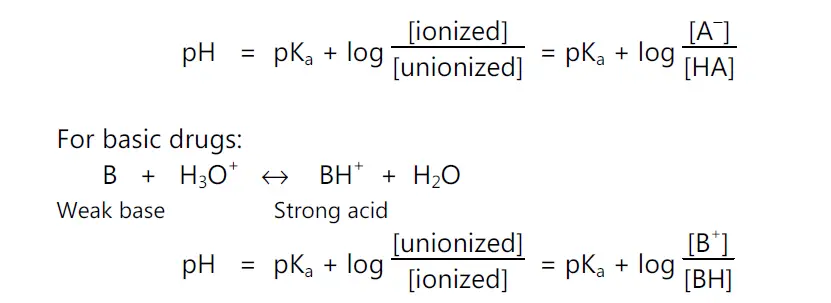
• pKa Determination: The Henderson – Hasseslebach equation provides an estimate of the ionized and unionized drug concentration at a particular pH.
For acidic drugs,
pH = pKa + log (ionized drug / un-ionized drug)
For basic drugs,
pH = pKa + log (unionized drug / ionized drug)
• Buffers, temperature, ionic strength and cosolvent can affect the pKa value.
• Potentiometric titration offers maximum sensitivity for compounds with pKa values in the range of 3-10.
Solubilization:
• Solubilization is increased by cosolvent addition.
• E.g. Propylene glycol solubilizes drug molecules by disrupting the hydrophobic interactions of water.
More non-polar the solute – Greater is the solubilisation achieved by cosolvent addition
Particle size:
• Fine particle characterization is very important property and here smallest particle should be tested to facilitate homogeneous sample preparation.
• Coulter Counter Technique: To check particle size and particle volume.
• BET (Brunauer, Emmet, Teller) nitrogen absorption apparatus: Measurement of surface area.
• SEM (Scanning Electron Microscopy): To check surface morphology.