Particle Size Determination Methods:
1. Sieving
2. Microscopy
3. Sedimentation rate method
4. Light energy diffraction
5. Laser holography
6. Cascade impaction
1. Sieving method:
• Range: 50 – 150 μm
• Simple, inexpensive
• If powder is not dry, the apertures get clogged.
2. Microscopy:
• Range: 0.2 – 100 μm
• Particle size can be determined by the use of calibrated grid background.
• Most direct method.
• Slow and tedious method.
3. Sedimentation method:
• Range: 1 – 200 μm
• Andreasen pipette is used.
4. Cascade impaction:
• The principle that a particle driven by an airstream will hit a surface in its path, provided that its inertia is sufficient to overcome the drug force that tends to keep in it in airstream.
5. Light energy diffraction:
• Range: 0.5 – 500 μm
• Particle size is determined by the reduction in light reaching the sensor as the particle, dispersed in a liquid or gas, passes through the sensing zone.
• Quick and fast.
6. Laser holography:
• Range: 1.4 – 100 μm
• A pulsed laser is fired through an aerosolized particle spray & photographed in three dimensional with holographic camera, allowing the particles to be individually imaged & sized.
• Particle size is characterized using these terms:
− Very coarse (#8)
− Coarse (#20)
− Moderately coarse (#40)
− Fine (#60)
− Very fine (#80)
Powder Flow Properties:
Flowability of powder and chemical stability depends on the habit and internal structure of a drug.
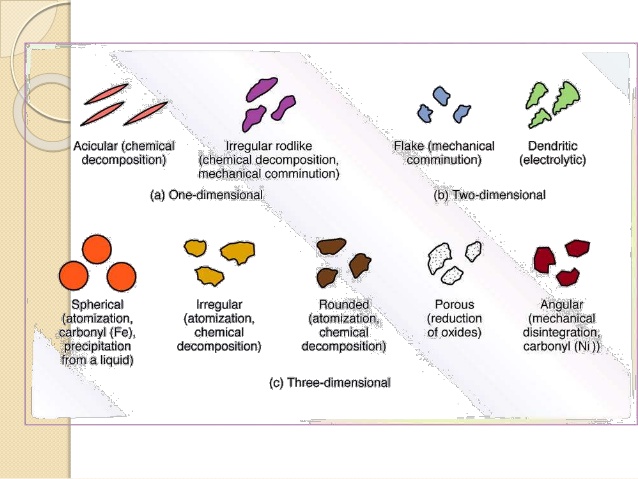
Habit is the description of the outer appearance of a crystal. A single internal-structure for a compound can have several different habits, depending on the environment for growing crystals. Different habits of crystals are given below.
• Powder flow properties can be affected by change in particle size, shape and density.
• The flow properties depend upon following-
(i) Force of friction.
(ii) Cohesion between one particle to another.
• Fine particle possesses poor flow by filling void spaces between larger particles causing packing & densification of particles.
• By using glidant we can alter the flow properties.
• e.g. Starch, Talc.
Determination of Powder Flow Properties:
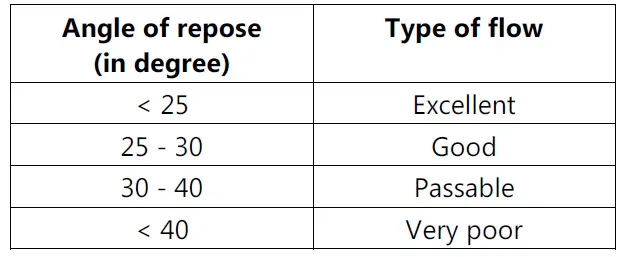
• By determining Angle of Repose.
• A greater angle of repose indicates poor flow.
• It should be less than 30° and can be determined by following equation:
tan θ = h / r
where, θ = angle of repose.
h = height of pile.
r = radius.
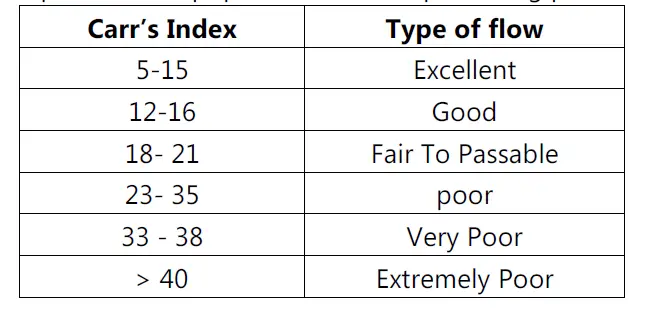
Measurement of free-flowing powder by compressibility:
• Also known as Carr’s index.
Car’s index (%) = (Tapped density − Poured density) / Tapped density × 100
Tapped Density:
• It is simple, fast and popular method of predicting powder flow characteristics.
Particle Shape:
• Particle shape will influence the surface area, flow of particles, packing & compaction properties of the particles.
• A sphere has minimum surface area per unit volume.
• Therefore, these properties can be compared for spheres & asymmetric particles, in order to decide the shape.
Surface Area:
• Particle size and surface area are inversely related to each other.
• Smaller the drug particle, greater the surface area.
• Specific surface is defined as the surface area per unit weight (Sw) or unit volume (Sv) of the material.
• However size reduction is not required in following cases when drug is unstable.
• Degrade in solution form.
• Produce undesirable effects.
• When sustained effect is desired.
Solubility Analysis:
• Preformulation solubility studies focus on drug solvent system that could occur during the delivery of drug candidate.
• e.g. A drug for oral administration should be examined for solubility in media having isotonic chloride ion concentration and acidic pH.
• Analytic methods that are particularly useful for solubility measurement include HPLC, UV spectroscopy, Fluorescence spectroscopy and Gas chromatography.
• Reverse phase HPLC offer accurate and efficient means of collecting solubility data of drug.
For determination of solubility the following points should be considered:
• The solvent & solute must be pure.
• A saturated solution must be obtained before any solution is removed for analysis.
• The method of separating a sample of saturated solution from undissolved solute must be satisfactory.
• The method of analyzing solution must be reliable
• Temperature must be adequately controlled.
• General Method of Increasing
• the Solubility
• Addition of co-solvent
• pH change method
• Reduction of particle size
• Temperature change method
• Addition of Surfactant
• Complexation
• Applications of solubilization
• Drugs with limited aqueous solubility can be solubilized. These include oil-soluble vitamins, steroid hormones and antimicrobial agents etc.
• Both oil-soluble and water-soluble compounds can be combined in a single phase system as in case of multivitamin preparations.
• Solubilization may lead to enhanced absorption and increased biological activity.
• Drug absorption from ointment bases and suppositories also increased.
• Aqueous concentrates of volatile oils can be prepared by solubilization.
• Example: soaps used for solubilising phenolic compounds for use as disinfectants-Lysol, Roxenol etc.
• Barbiturates, anticoagulant, alkloidal drugs are dissolved with polysorbate by solubilization.
Formulation Challenges with Poorly Soluble Compounds:
• Poor dissolution rate
• Low and variable bioavailability
• More potential for food effect
• Inability to deliver high doses for toxicity studies
• Difficulty in developing parenteral formulations Stability:
• Stability is the extent to which a product retains (throughout its period of storage and use, i.e., its shelf life) the same properties that it possessed at the time of its manufacture.
• One of the principles of dosage form design is to ensure that the chemical integrity of drug substances is maintained during the usable life of the product.
• Three types of stability concern the pharmacists:
(i) Chemical: Each active ingredient retains its chemical integrity within the specified limits.
(ii) Physical: The original physical properties (including appearance, taste, color and odor) are retained.
(iii) Biological: Sterility is retained (No microbial growth).