Auto Coater 48’’ GMP Model
Auto Coater 48’’ GMP Model –The purpose of the Tablet Coating System is to uniformly coat tablets by spraying them with coating materials in a controlled environment & on a moving tablet bed. The hot air entering this equipment will enable efficient drying of tablets, while they are being coated. The complete system can be divided into the following sub-sections:
- Inlet Air Handling Unit
- Pan Housing (Inlet Air Plenum, Exhaust Air Plenum, Pan & Spraying System)
- Exhaust Air Handling System
Inlet Air Handling Unit: It consists of pre-air filters, HEPA filters, an Air heating unit, and airflow & temperature measuring devices. The inlet air handling unit draws air, with the help of a blower, from the environment processes the inlet air, and controls the inlet airflow. It also contains the Dehumidification section. Dehumidification coils are provided in AHU for RH control.
Pan Housing (48” Pan): The pan housing consists of a rotating pan, with perforation on its periphery. This perforated surface allows the hot air to pass through. The pan is rotated via a motor gearbox drive with suitable sealing. A temperature measuring device is also mounted to measure the temperature of the bed in the pan.
The pan has baffles that help the tablets to move in a very uniform pattern, thereby receiving a uniform coating. The spray system consists of a Solution vessel with a Pneumatic stirrer, a Peristaltic Pump & the spray nozzle assembly. Silicone tubes (Food Grade) are used to transfer material from the solution vessel to the spray nozzle, via the peristaltic pump. The atomization of the spray solution is achieved by providing high-pressure compressed air to the atomizing spray nozzles. The spray rate & the atomizing pressure can be controlled separately. The Spray rate can be controlled by varying the RPM of the Peristaltic Pump.
Auto coater is an automated tablet coating system for efficient film coating of tablets with cGMP compliance in closed conditions. The main Pan unit consists of a cylindrical perforated pan with conical ends in an SS double–walled enclosure. Tablets to be coated are charged into the Pan. During the coating process, coating fluid is sprayed (Film Coating) through multiple air-borne spray Gun (s) mounted within the pan. A Peristaltic pump is employed for the precise delivery of coating fluid. The Tablet bed is gently and efficiently mixed during pan rotation with the aid of mixing baffles attached internally, within the pan. The coated tablet cores are dried with heated, air supplied from inlet AHU which contains a heating system as well as a Sequential battery of EU-7, EU-9 & EU-13 µ filters. As a result, the applied coating is dried with non-contaminated, dust-free, and optimized volumes of air, for producing uniformly coated tablet cores.
The air handling system is modularly constructed to allow quick and easy removal of sub-assembly for maintenance and repairs. Pre–clarified air is drawn through an inlet blower for dehumidification through a chilled water coil & heating through a steam coil. Heating air is conveyed through the discrete filter cabinet containing 5µ secondary filters.
Exhaust air is passed through a wet scrubbing system, to minimize expulsion of dust & coating resins from coating systems, comprised of MS scrubber with inlet and outlet connections, fed through suitable piping by a pump from the water holding tank. An electrically operated peristaltic pump is incorporated outside the unit. The silicon tubing passes through the pump, of which one end is dipped into the solution tank, and the other end is connected to the coating/or dosing manifold. W.I.P System consisting of strategically mounted high capacity spray nozzles fed through pipe network from the pump with the solenoid. Strong jets are provided diagonally opposite on the left & right sides of the perforation area of the pan. Nozzles are also provided for washing front and back cones.
Performance Qualification Protocol Content for Auto Coater
- Approval
- Objective
- Responsibility
- System Description – 1.Equipment Identification 2.Description of Operation
- Standard Operating Procedure Established During Operation Qualification
- Validation Plan and Methodology
- Performance Qualification Procedure
- Gun Distance from Bed
- RPM of Peristaltic pump and Spray Rate
- Effect Of Pan Load
- Effect Of Inlet Temperature
- Effect Of Atomizing Pressure
- Negative Pressure in PAN
- Effect of PAN R.P.M
- Uniformity of Weight Build-Up
- Deviation and Corrective Action
- Change Control Proposal
- Revalidation Criteria
- Acceptance Criteria
- Evaluation of Result
- Conclusion
Objective: The objective of this protocol is to collect sufficient data to establish that Performance Qualification for Auto Coater 48’’ supplied by ____________________. Performs to meet the desired Product Quality consistently, when operated as per Standard Operating Procedure.
Performance Qualification Protocol shall provide the Methodology of qualification studies, formats for recording the observation, Criteria of Qualification, and a guideline for documentation of the study.
Responsibility: The validation group comprising a representative from each of the following departments shall be responsible for the overall compliance with this protocol:
- Production Department
- Quality Assurance Department
- Quality Control Department
The Production department shall be responsible for checking the operations and recording data as per the procedures outlined in the data sheets of this protocol. The Quality control department shall be responsible for testing samples wherever required.
The Protocol of Performance qualification shall be initiated and Checked by the Production Department. The report shall be finally authorized by the GM operation and Quality Assurance head.
Equipment Identification: The subjected equipment is identified as Auto Coater
The Equipment is identified as Auto Coater 48’’ GMP MODEL
Model No. : GMP Model
Description of Operation: The film coating Machine consists of a Cylindrical-horizontally placed pan having a tablet bed in an enclosed system controlled with a variable speed motor. It is provided with a wide round mouth to incorporate the Triple spray gun assembly for spraying the coating solution through the peristaltic pump and PLC system.
Standard Operating Procedure established during Operation Qualification
- Title: Auto Coater (Eq. ID.
- Operation of Auto Coater
- Cleaning of Auto Coater
- Preventive maintenance of Auto Coater
Validation Plan and Methodology:
Performance of the Tablet Coating Auto Coater 48’’ GMP Model shall be tested at extreme and optimum operational conditions, which are identified as Critical Process Variables. Critical Process Variables are:
- Gun distance from tablet bed
- Spray rate
- Pan load
- Inlet Temperature
- Atomizing air pressure
- Negative pressure in the pan
- Pan RPM
- RPM of Peristaltic
Equipment Performance Qualification shall be carried out, simulating actual production conditions and varying minimum & maximum critical process variable conditions.
No Pharmaceutical Active Ingredients shall be used for the qualification purpose. Placebo tablets and film coating excipients are used for manufacturing routine products using aq. coating method shall be used for the study. The composition of the placebo and method for film coating is described in an Approved Batch Production Record attached as Attachment #01
In-process testing shall be carried out for verification of physical appearance (Sticking, picking, capping, shade variation), shape, and embossing/engraving. A minimum of three trials shall be carried out for each process variable at three points, to verify the consistency and reproducibility of the equipment performance. The Validation Plan is described in the following Matrix.
Process Variable
- Gun distance from tablet bed (cm)
- Spray rate (g/min.)
- Pan load (Kg)
- Inlet Temperature (°C)
- Atomizing Air pressure (Kg. / cm2)
- Negative pressure in pan (mm)
- Pan RPM
- RPM of Peristaltic pump
Observed Parameters for qualification= 18/22/24
Number of trials = 01 for each qualification parameter
Quality Parameters to be tested =Appearance of tablet surface during coating.
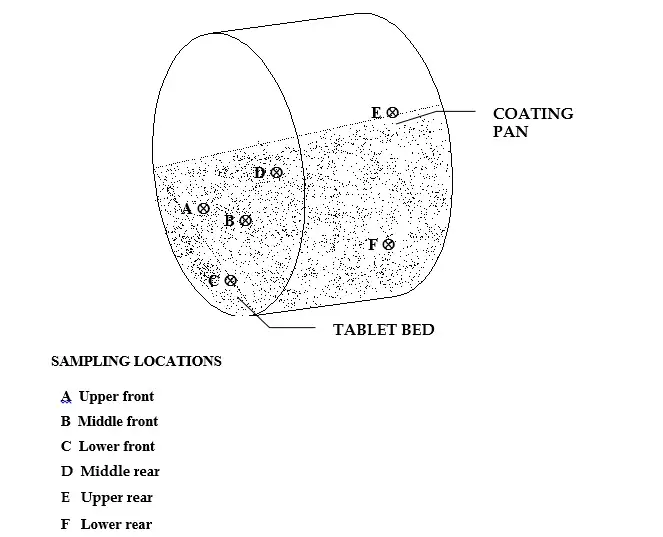
Sampling frequency: 20 tablets from 6 locations of the pan.
Performance Qualification Procedure:
Test Data Sheet
Date: …………….. Time: ……………..
Gun Distance from Bed
Gun Distance from Tablet Bed (cms) = 18/22/24
Bed Temperature (°C)
- Picking
- Sticking
- Surface Roughness
- Non-Uniformity of Colour
- Uniform Coating
RPM of Peristaltic pump and Spray Rate.:
Test Data Sheet /Date: ……………../Time: ……………..
RPM of peristaltic pump = 18/22/26
Observed Spray Rate (g / Min.) = Gun1 /Gun2 /Gun3 ,Total =________
Bed Temperature (°C)
The appearance of the Tablet Surface (Tick ü whichever is applicable)
- Picking
- Sticking
- Surface Roughness
- Non-Uniformity of Colour
- Uniform Coating
Effect Pan Load
Test Data Sheet /Date: ……………../Time: ……………..
Pan Load (Kg.) = 40.0 kg/60.0 kg /80.0 kg
Appearance of Tablet Surface (Tick ü whichever is applicable)
- Picking
- Sticking
- Surface Roughness
- Non-Uniformity of Colour
- Uniform Coating
Bed Temperature (°C)
Effect of Inlet Temperature
Test Data Sheet /Date: ……………../Time: ……………..
Inlet Temperature ( 50/55/60°C ) / Bed Temperature (°C)
Appearance of Tablet Surface (Tick ü whichever is applicable)
- Picking
- Sticking
- Surface Roughness
- Non-Uniformity of Colour
- Uniform Coating
Effect of Atomizing Pressure
Test Data Sheet / Date: ……………../Time: ……………..
Atomizing Pressure (Kg. / cm2) = 2.0 /2.5 /3.0 / Bed Temperature (°C)
Appearance of Tablet Surface (Tick ü whichever is applicable)
Picking /Sticking /Surface Roughness /Non Uniformity of Colour /Uniform Coating
Negative Pressure in Pan:
Test Data Sheet / Date: ……………../Time: ……………..
Effect of Pan R.P.M:
Test Data Sheet Date: ……………../Time: ……………..
Pan RPM /Bed Temperature (°C)
Appearance of Tablet Surface (Tick whichever is applicable)
- Picking
- Sticking
- Surface Roughness
- Non-Uniformity of Colour
- Uniform Coating
Uniformity of Weight Build-Up:
Test Data Sheet Date: ……………../ Time: ……………..
Initial Pre Warm Average Weight of core: ( 100 Tablets)
Sampling Locations in Pan/Weight Observed (gm.)100 Tablets/Average Weight (mg.)/% Actual Weight Build Up
% Actual Weight Build Up
Film Coating Efficiency:————————- X 100 = % Theoretical Weight Build Up
Sampling Locations are shown diagrammatically below
Deviation and Corrective Action
Description of deviation and date observed,The person responsible for corrective action and the date assigned , Corrective action taken, and date conducted
Re-Validation Criteria
- Performance Qualification of Auto Coater to be re-qualified on:
- Substitution of existing Auto Coater with a new Auto Coater.
- Replacement of existing instrument/component with a new one, which can have a direct impact on the performance of the Auto Coater.
- Any major modification to the existing Auto Coater can affect the performance of the equipment.
- If the Auto Coater is found to be malfunctioning during performance qualification.
Acceptance Criteria
Performance Qualification shall be considered acceptable when all the conditions specified in respective data sheets are completed and optimum process conditions are finalized, with the simulation of actual production conditions and varying minimum & maximum critical process variable conditions.
Any deviation from the acceptance criteria of the specific checkpoint shall be reported and a decision will be taken for the rejection, replacement, or rectification of the equipment/component.
Evaluation of Result:
Results shall be documented in Test Data Sheets based on the observations recorded in Performance Qualification. Evaluation of results shall be carried out by finalizing the operational range of equipment for processing of products.
All test results meeting the Acceptance Criteria shall establish the satisfactory performance of Auto Coater when operated as per SOP.
Conclusion: A summary report shall be prepared to summarize the results of the PQ test . Based on results and evaluation, a conclusion shall be drawn to state the adequacy of the equipment to produce Quality Products meeting the desired Product Quality consistently, when operated as per Standard Operating Procedure.
Abbreviations
Min – Minutes
RSD – Relative Standard Deviation
SOP – Standard Operating Procedure
PQ – Performance Qualification
RPM – Rotations Per Minute
QA – Quality Assurance
G – Grams
FDA – Food & Drug Administration
References
Protocol Format and Style Guide – FDA guidelines
Annexure
Q.C. Test report