MASTER MANUFACTURING FORMULA OF LISINOPRIL & HYDROCHLOROTHIAZIDE 10+12.5 MG TABLETS
Contains
Label Сlaim
Manufacturing Formula
List of Equipment
Manufacturing Instructions
Manufacturing Process Flow Chart
Manufacturing Process Details
Packaging
Finished Product Specifications
Procedural Changes
Label Сlaim
| Product | Lisinopril & Hydrochlorothiazide Tablets |
| Strength | 10+12.5 mg |
| Label сlaim | Each tablet contains:
Lisinopril dihydrate equivalent to Lisinopril………10 mg Hydrochlorothiazide ……………………………..12.5 mg |
| Shelf life* | 24 months |
| Storage | Store at temperature below 25° C. Protect from light. |
| Category | Antihypertensive & Diuretics |
* The shelf life is tentative and shall be finalized based on stability studies.
2.0 Manufacturing Formula
| Sr. No | Ingredients | Grade | Supplier | Category | Quantity per Unit (mg) | Batch Qty.(in Kg) |
| Intragranular | ||||||
| 1. | Lisinopril dihydrate*
(equivalent to Lisinopril) |
EP | – | API | 10.890
(10.000) |
2.72 |
| 2. | Mannitol (Pearlitol 200 SD) | USP | – | Diluent | 16.500 | 4.13 |
| 3. | Calcium Hydrogen Phosphate Dihydrate | USP | – | Diluent | 45.000 | 11.25 |
| 4. | Maize Starch B | EP | Roquette Freres | Binder | 16.500 | 4.13 |
| Binder solution | ||||||
| 5. | Pregelatinised starch (Starch 1500) | BP | Colorcon | Binder/
Disintegrant |
3.300 | 0.83 |
| 6. | Purified Water | USP | – | Solvent | Q.S.
|
20.67 |
| Extragranular | ||||||
| 7. | Colloidal Silicon dioxide (Aerosil 200 Pharma) | BP | – | Glidant | 0.550 | 0.14 |
| 8. | Hydrochlorothiazide (non-micronized)* | EP | API | 12.500 | 3.13 | |
| 9. | Mannitol (Pearlitol 200 SD)*** | USP | – | Diluent
|
3.660 | 0.92 |
| 10. | Magnesium Stearate | BP | – | Lubricant | 1.100 | 0.28 |
| Tablet weight | 110.000 | 27.50 | ||||
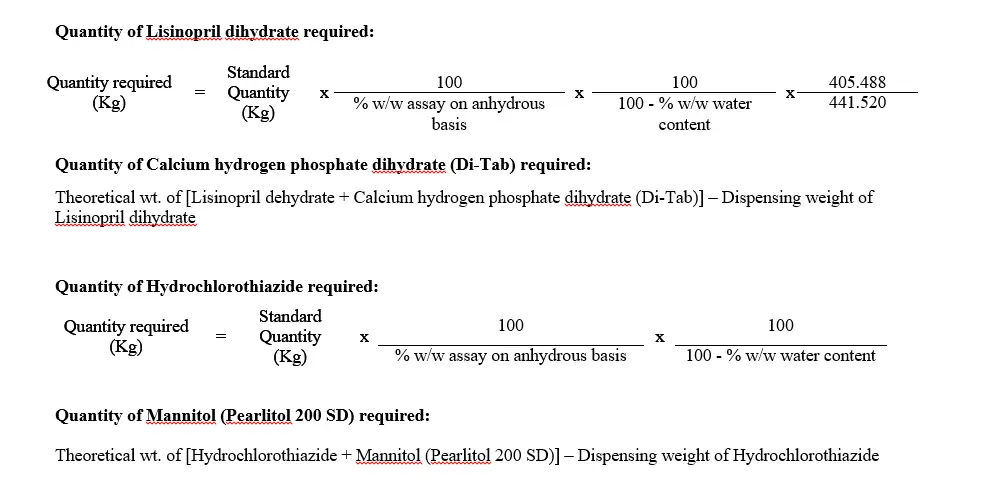
* The quantity is based on 100% w/w assay value (on anhydrous basis) & nil water content of Lisinopril dihydrate & Hydrochlorothiazide.
** Quantity of Calcium hydrogen phosphate dihydrate (Di-TAB) to be adjusted based on actual assay & % w/w water content of Lisinopril dihydrate.
*** Quantity of Mannitol (Pearlitol 200 SD) to be adjusted based on actual assay & % w/w water content of Hydrochlorothiazide.
List of Equipment
| Sl. No. | Equipment Name# |
| 1. | Sifter |
| 2. | Fluid Bed Processor |
| 3. | Multimill |
| 4. | Conta Blender |
| 5. | Compression Machine |
| 6. | Blister Packing Machine |
# Latest versions of SOPs’ shall be followed for operating, cleaning, calibration and maintenance of all equipment.
Manufacturing Instructions:
- Ensure machine guards are in place before starting the operations.
- Maintain raw material and finished product at temperature 20 – 25 °C and relative humidity 50 ± 20 %. The same environmental conditions should be maintained during manufacturing.
- The following ‘Personal Protective Measures’ are to be taken: When working with the active substance, drug products or with mixtures of the active substance and excipients wear latex gloves and dust masks to avoid exposure and contact to any body parts.
General Precautions:
- Therapeutic category: Antihypertensive agent (Lisinopril) and Diuretics (Hydrochlorothiazide).
- Before working with Lisinopril & Hydrochlorothiazide, read Material Safety Data Sheet of the active ingredient.
- Avoid contact with skin and eyes.
- General reported side effects: unexplained muscle pain, tenderness, or weakness; confusion, memory problems; fever, unusual tiredness, and dark colored urine.
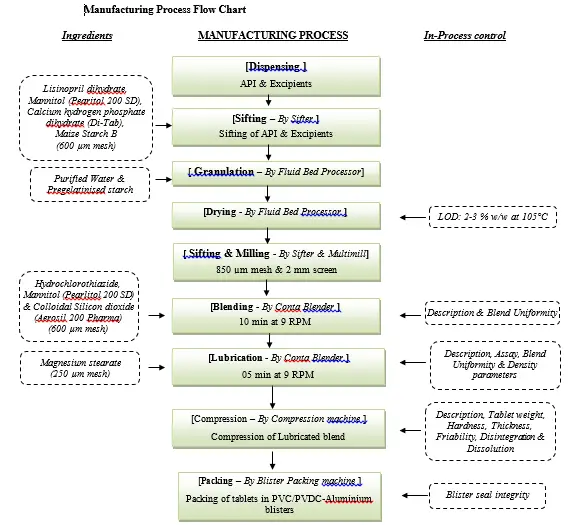
Manufacturing Process Details
- Sifting of Intragranular excipients :
- Sift the dispensed quantity of Lisinopril dihydrate, Mannitol (Pearlitol 200 SD), Calcium hydrogen phosphate dihydrate (Di-Tab) & Maize Starch B together through 600 µm mesh (ASTM, # 30 sieve) and keep in a separate prelabelled polybag.
Dry Mixing:
- Load the materials of step 6.1.1 in Fluid Bed Processor and fluidize for 10 minutes.
- Preparation of Binder solution
- Take 15.56 Kg of Purified Water in a stainless steel container.
- Add Pregelatinised Starch (Starch 1500) slowly into Purified water under stirring. Continue stirring for 15 minutes.
- Granulation
- Spray the binder solution to the dry mix as per the following parameters given below:
| Parameters | Limits |
| Inlet temperature | 25 – 65°C |
| Bed temperature | 25 – 45°C |
| Blower speed | 16 – 40 Hz |
| Peristaltic pump speed (Spray Rate) | 20– 50 rpm |
| Loss on drying (LOD) | 2.0 – 3.0% |
- Granulate the materials by spraying the binder with continuous drying. After completion of the binder solution, observe the uniformity of granules visually.
- If required, rinse the binder solution container with of Purified water & continue spraying on to the granules with the parameters mentioned .
Drying:
- Dry the wet mass at an inlet air temperature of 60°C± 5°C to get LOD in the range of 2 to 3% w/w at 105°C in auto mode using IR moisture analyzer.
Sifting and Milling :
- Sift the dried granules of step 6.5 through 850 µm mesh (ASTM, # 20 mesh).
Mill the retentions of step 6.6.1 through Multimill fitted with 2.0 mm screen at slow speed.
- Sifting of Extragranular Materials :
- Sift Hydrochlorothiazide, Mannitol (Pearlitol 200 SD) & Colloidal Silicon dioxide (Aerosil 200 Pharma) together through 600 µm screen (ASTM, #30 sieve).
Sift Magnesium Stearate through 250 µm screen (ASTM, #60 sieve).
Blending :
- Pre-Mixing
Load the above Milled materials and sifted Extragranular Materials in Conta blender and mix for 10 minutes at 9 RPM.
Final Mixing
Load the above sifted Magnesium Stearate into the blender and mix for 5 minutes at 9 RPM.
- Unload the lubricated blend into double lined polybag in suitable containers.
Blend Sample Analysis :
Collect samples as per sampling plan as per flow diagram, send the blend samples to QC for analysis and check according to the table mentioned below:
| In process specification for lubricated blend | ||
| Test | Specification | |
| Appearance of blend | White to off white free flowing granules | |
| Blend uniformity | 90% to 110% of label claim with RSD of NMT 5% | |
| Blend assay | 95% to 105.0% of label claim | |
-
Compression:
- After receiving QC approval for blend, compress the blend by compression machine as per the following parameters under controlled environmental condition.
| Parameters | Lisinopril & Hydrochlorothiazide Tablets | |
| Tooling | Size | 6.00 mm |
| Shape | Round | |
| Embossing | Upper Punch | Plain |
| Lower Punch | Plain | |
| Description | White to off-white, round, flat, uncoated tablet ,plain on both side. | |
| Diameter (mm) | 6.0 ± 0.1 (5.9 – 6.1) | |
| Average mass of 20 tablets (g) | 2.20 ± 5.0 % (2.09 – 2.31) | |
| Uniformity of Weight (mg) | 110.00 ± 7.5% (101.75 – 118.25) | |
| Hardness (kP) | 2.0 – 10.0 | |
| Parameter | Lisinopril & Hydrochlorothiazide Tablets |
| Thickness (mm) | 2.65 ± 0.2 (2.45 – 2.85) |
| Disintegration Time (minutes) | NMT 15 at 37°C in water |
| Friability (% w/w) | NMT 1.0% |
Collect the compressed tablets in double polythene bag lined and labeled cleaned containers.
Packaging :
After receipt of QC testing release, pack the tablets in PVC/PVDC-Aluminium blister pack.
Details of Primary Packaging material are mentioned below:
| Parameters | Lidding Foil (Aluminium) | Forming Film
Clear PVC/PVdC (250/60) (250/60) |
Pack Size | Forming Temperature | Sealing Temperature
(°C) |
| Thickness (micron) | 25 | 286 | 2 or 6 blisters of
14 tablets
#28 (14×2) #84 (14×6) |
140 to 160 | 180 to 200 |
| Width (mm) | 180 | 184 | |||
| Grammage (GSM) / | 75 ± 5 % | 400 ± 7 % |
Finished Product Specifications
Analyze the finished product samples as per Current Standard Testing Procedure.
Procedural Changes
If a production department has compelling reasons for modifying this manufacturing procedure, it should proceed only after approval from Research & Development and Quality Assurance department.