All Post URL of Drugs formulations Tablets MFR of Albendazole Tablets – (drugsformulations.com) MFR of Alfacalcidol & Calcium Carbonate Tablets (drugsformulations.com) MFR of Sildenafil Citrate Tablets (drugsformulations.com) MFR of Paracetamol & Dicyclomine HCl Tablets – (drugsformulations.com) MFR of Dextromethorphan, Bromhexine, Chlorpheniramine Maleate & Guaifenesin Tablets (drugsformulations.com) Dextromethorphan (drugsformulations.com) Spiramycin Tablet (drugsformulations.com) MFR of Propranolol Hydrochloride …
Read More »Sterilization (Introduction, Methods, Definition of Terms)
Sterilization (Introduction, Methods, Definition of Terms) Introduction A major risk of all such medical and surgical instruments procedures is the introduction of pathogens that can lead to infection. Failure to properly disinfect or sterilize equipment carries not only risk associated with breach of host barriers but also risk for person-to-person …
Read More »Technology Transfer in pharmaceutical manufacturing (WHO)
Technology Transfer in pharmaceutical manufacturing (WHO) Introduction Scope Glossary Organization and management Production: transfer (processing, packaging and cleaning) Quality control: analytical method transfer Premises and equipment Documentation Qualification and validation 1.1 Transfer of processes to an alternative site occurs at some stage in the life-cycle of most products, from development, …
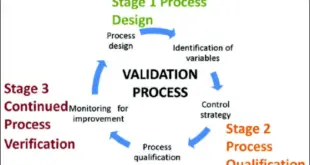
Read More »Process Validation Critical Parameters
Process Validation Critical Parameters Process Validation(FDA Definition) Establishing Documented Evidence, Which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.” Steps in Validating a Process: Develop validation protocol Conduct installation qualification Conduct operational qualification Conduct performance qualification …
Read More »Equipment Status log
Equipment Status log OBJECTIVE: To lay down a procedure of the maintenance of equipment sequential log. SCOPE: This SOP is applicable to all equipment and machines of all manufacturing departments RESPONSIBILITY: Production and Engineering officer/above shall be responsible to follow the procedure mentioned in this SOP. ACCOUNTABILITY : Concerned Department Head and QA Head …
Read More »Cleaning of general item
Cleaning of general item OBJECTIVE: To lay down a procedure to describe the steps to be followed for cleaning general items. SCOPE: Applicable for cleaning all HDPE / SS containers, utensils, scoops, sampling tools and different cleaning aids RESPONSIBILITY: Housekeeping workers, Production Supervisors, Operators, IPQA personnel shall be responsible to follow the …
Read More »NON-CONFORMANCE RESULTS
NON-CONFORMANCE RESULTS OBJECTIVE: To lay down the procedure for define the necessary action against the non-conformance of a product. SCOPE: This procedure is applicable for information, investigation and control for any kind of non- conformance noticed or confirmed in Production, Stores on in others area for its proper investigation RESPONSIBILITY: Officer – …
Read More »Prevention and Product Mix Up
Prevention and Product Mix Up OBJECTIVE: To lay down the Procedure for prevention of Product mix-up. SCOPE: This procedure is applicable for the control and prevention of product mix up during and after the manufacturing of the product . RESPONSIBILITY: QA officer and Production Officer shall be responsible for the …
Read More »SOP on house keeping, cleaning and sanitization of general area in Pharmaceutical company
SOP on housekeeping, cleaning, and sanitization of the general area in a Pharmaceutical company. Objective: To lay down a procedure for Good House Keeping, cleaning, and sanitization of the General Area. Scope: This procedure is applicable for cleaning and sanitization of General areas other than the manufacturing area of the …
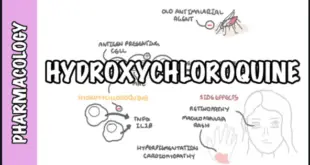
Read More »Hydroxychloroquine and Antimalarial Drugs (united healthcare)
Hydroxychloroquine and Antimalarial Drugs (united healthcare) These are antimalarial drugs found to induce remission in upto 50% patients of Rheumatoid Arthritis (RA), but take 3–6 months. Their advantage is relatively low toxicity, but efficacy is also low; bony erosions are not prevented. Their mechanism of action is not known, however, …
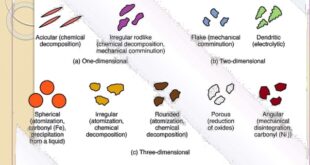
Read More »Characterization of Fine Particle in Formulation of drugs
Characterization of Fine Particle in Formulation of drugs Parameters those are measured: (i) Particle size and size-distribution (ii) Shape of the particle (iii) Surface morphology of the particles (iii) Zeta potential Instrumental Methods of Particle Size Characterization: (i) Light Microscope: • First a standard graticule (BS 3625) is standardized with …
Read More »PROCESSING OF PARENTERAL PREPARATION
PROCESSING OF PARENTERAL PREPARATION The following steps are involved in the processing of parenteral preparation: 1. Cleaning of containers, closures, and equipment. 2. Collection of materials. 3. Preparation of parenteral products. 4. Filtration. 5. Fill the preparation in the final container. 6. Sealing the container. 7. Sterilization 8. Evaluation of …
Read More »Formulation of Suspensions
Formulation of Suspensions Biphasic liquids such as suspensions and emulsions are unique dosage forms because many of their properties are due to the presence of a boundary region between two phases. In suspensions, a liquid and an insoluble solid meet to form an interface. In the case of emulsions, two …
Read More »Determination of equilibrium solubility of a drug
Determination of equilibrium solubility of a drug Determination of equilibrium solubility of a drug: The drug is dispersed in a solvent. The suspension is agitated at a steady temperature. Samples of the suspension are withdrawn as a function of time, clarified by centrifugation,and assayed to establish a plateau concentration. Solvents …
Read More »PROBLEMS AND REMEDIES FOR TABLET COATING
PROBLEMS AND REMEDIES FOR TABLET COATING Blistering It is local detachment of film from the substrate forming blister. Reason: Entrapment of gases in or underneath the film due to overheating either during spraying or at the end of the coating run. Cause and Remedy of Blistering: Cause: Effect of temperature …
Read More »VALIDATION AND QUALIFICATION OF WATER PURIFICATION,STORAGE,AND DISTRIBUTION SYSTEMS AS PER USP
Purified Water Establishing the Quality of pharmaceutical water purification,storage,and distribution systems requires an appropriate period of monitoring and observation. However, it is more difficult to meet established microbiological quality criteria consistently. A typical monitoring and observation program involves intensive daily sampling and testing of major process points for at least …
Read More »VITAMINS AND MINERALS CHART
VITAMINS AND MINERALS CHART Different types of vitamins and minerals enable healthy body function, such as cell and tissue repair, production of cells, and healthy brain function. Your body doesn’t produce these vitamins and minerals on its own, and while you can get most of these from a supplement, your …
Read More »Lubricants and Glidants used in Tablet Formulations
Tablets Lubricants Lubricants and Glidants used in Tablet Formulations are: • Magnesium stearate • Stearic acid • Sodium stearyl fumarate • Hydrogenated vegetable oil • PEG 4000, 6000 • Hexagonal boron nitride • DL-Leucine • Sodium lauryl sulfate • Gliceryl behenate • Sodium benzoate • Colloidal silicone dioxide • Talc …
Read More »Preformulation stability studies
Stability Analysis Preformulation stability studies are the first quantitative assessment of chemical stability of a new drug. This may involve 1. Stability study in toxicology formulation 2. Stability study in solution state 3. Stability study in solid state. Stability Study in Toxicology Formulation: A new drug is administered to animals …
Read More »Powder Particle Size Determination Methods for tablets
Particle Size Determination Methods: 1. Sieving 2. Microscopy 3. Sedimentation rate method 4. Light energy diffraction 5. Laser holography 6. Cascade impaction 1. Sieving method: • Range: 50 – 150 μm • Simple, inexpensive • If powder is not dry, the apertures get clogged. 2. Microscopy: • Range: 0.2 – …
Read More »