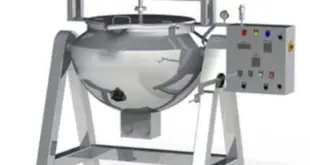
SOP on operation of Paste Kettle Standard Operating Procedure (SOP) Objective To lay down the procedure for operation of Paste Kettle Scope This SOP is applicable for operation of Paste Kettle in the formulation plant. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ Executive – To …
Read More »Tablets In Pharma Industry
Tablets In Pharma Industry NOTE- The provisions of this monograph do not necessarily apply to tablets intended for use other than by oral administration such as Vaginal preparations or Or mucosal preparations, and to lozenges, oral pastes and oral gums. Introduction Tablets are solid dosage forms each containing a unit …
Read More »CAPSULES IN PHARMA INDUSTRY
CAPSULES IN PHARMA INDUSTRY Capsules are solid dosage forms in which the drug substance is enclosed within either a hard or soft soluble shell. Generally, the shells are formed from gelatin. The capsule may be regarded as “container” drug delivery system, which provides a tasteless/odorless dosage form without the need …
Read More »Disintegration Test in Pharma Industry
Disintegration Test in Pharma Industry This test determines whether dosage forms such as tablets, capsules, boluses pessaries, and suppositories disintegrate within a prescribed time when placed in a liquid medium under the prescribed experimental conditions. For the purpose of this test, disintegration does not imply complete solution of the dosage …
Read More »SOP for cleaning of FBD bag
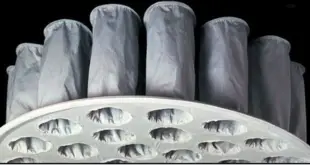
SOP for cleaning of FBD bag Standard Operating Procedure (SOP) Objective To lay down a procedure for cleaning of FBD bags. Scope This SOP is applicable for the cleaning of FBD bags in the formulation plant. Responsibility Production Operator/ Technician – For cleaning of the equipment. Production Officer/ Executive – …
Read More »SOP for operation of Tablet Inspection Machine
SOP for operation of Tablet Inspection Machine Standard Operating Procedure (SOP) Objective To lay down the procedure for operation of Tablet Inspection Machine. Scope This SOP is applicable for the operation of Tablet Inspection Machine in the formulation plant. Responsibility Production Operator/ Technician – For operation of the machine. Production …
Read More »SOP for operation of Auto coater
SOP for operation of Auto coater Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of Auto coater. Scope This SOP is applicable for operation of Auto coater in the formulation plant. Responsibility Production Operator/ Technician – For operation of the machine. Production Officer/Executive -To maintain proper …
Read More »SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry
SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of rapid mixer granulator with cone mill in Pharma Industry Scope This SOP is applicable for operation of rapid mixer granulator with cone mill …
Read More »Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry
Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for cleaning of Rapid Mixer Granulator with cone mill in Pharma Industry. Scope: This SOP is applicable for the cleaning of Rapid Mixer Granulator with cone mill located in …
Read More »SOP on cleaning of Paste Kettle in Pharma Industry
SOP on cleaning of Paste Kettle in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for cleaning of Paste Kettle in Pharma Industry. Scope This SOP is applicable for the cleaning of Paste Kettle in the formulation plant of Pharma Industry. Responsibility Production Operator/ Technician – …
Read More »SOP for operation of Metal Detector in Pharma Industry
SOP for operation of Metal Detector in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for operation of metal detector in Pharma Industry. Scope: This SOP is applicable for the operation of metal detector to the formulation plant of Pharma Industry. Responsibility: Production Operator/ Technician shall be …
Read More »SOP on Operation of Paste Kettle In Pharma Industry
SOP on the operation of Paste Kettle In the Pharma Industry Objective To lay down the procedure for operation of Paste Kettle In Pharma Industry. Scope This SOP is applicable for operation of Paste Kettle In Pharma Industry. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ …
Read More »Contract production, analysis and other activities as per WHO Guideline in Pharma Industry
Contract production, analysis, and other activities in the Pharma Industry Principle. Contract production, analysis and any other activity covered by GMP must be correctly defined, agreed and controlled in order to avoid misunderstandings that could result in a product, or work or analysis, of unsatisfactory quality. All arrangements for contract …
Read More »Pharma Manufacturing Premises – Risk classification observations
Pharma Manufacturing Premises – Risk classification guide for drug good manufacturing practices observations Risk 1 (critical) observations in Pharma Industry There was no air filtration system to eliminate airborne contaminants likely to be generated during fabrication or packaging. There was generalized malfunctioning of the ventilation system(s), with evidence of widespread …
Read More »Process Validation: General Principles (USFDA) in Pharma Industry
Process Validation: General Principles (USFDA) in Pharma Industry This guidance incorporates principles and approaches that all manufacturers can use to validate manufacturing processes. FDA encourages the use of modern pharmaceutical development concepts, quality risk management, and quality systems at all stages of the manufacturing process lifecycle. The lifecycle concept links product …
Read More »Indian Pharmacopoeia Commission (IPC)
Indian Pharmacopoeia Commission (IPC) In the healthcare system, Indian Pharmacopoeia Commission (IPC) plays an important role particularly in matters related to the quality standards, safety and rational use of medicines in the country. IPC was set up on 01st January, 2009 as an autonomous Institution under the Ministry of Health …
Read More »SOP for Procedure of shutdown and startup of Facility
SOP for Procedure of shutdown and startup of Facility Objective To lay down the procedure of shutdown and start-up after shutdown of Facility. Scope This SOP is applicable for procedure of shutdown and start up after shutdown of Facility in the formulation plant. Responsibility All personnel working in respective areas. …
Read More »SCALE UP AND POSTAPPROVAL CHANGES (SUPAC) GUIDANCE FOR INDUSTRY: A REGULATORY NOTE – PHARMA
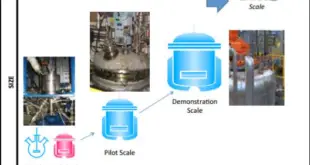
SCALE UP AND POSTAPPROVAL CHANGES (SUPAC) GUIDANCE FOR INDUSTRY: A REGULATORY NOTE – PHARMA INTRODUCTION Technology transfer of a pharmaceutical product from research to the production floor with simultaneous increase in production outputs is commonly known as scale-up. In simple terms, the process of increasing batch size is termed as …
Read More »SOP for PLASTICS AS PACKAGING MATERIAL
SOP for PLASTICS AS PACKAGING MATERIAL Plastics in packaging have proved useful for a number of reasons, including the ease with which they can be formed, their high quality, and the freedom of design to which they can be changed. Plastic containers are extremely resistant to breakage and thus offer …
Read More »SOP for Hold Time Studies Protocol of Ornidazole Tablets 500 mg
SOP for Hold Time Studies Protocol of Ornidazole Tablets 500 mg Process Step – Blending/ Lubrication About 500 gms material from blended mass is kept under simulating conditions. The material is subjected to analysis for Assay of Ornidazole,Total & single impurity and LOD at 0 day, after 7th day & after 15th …
Read More »