Cleaning of Factory Footwear Objective To lay down a procedure for Cleaning of Factory Footwear. Scope This Standard Operating Procedure is applicable for cleaning of factory footwear in the formulation plant. Responsibility Housekeeping personnel is responsible to follow the procedure mentioned in SOP. HR Department is responsible for implementation of …
Read More »Sterilization Process Controls
Sterilization Process Controls Inspectional Objectives Confirm that the sterilization process was validated by reviewing the validation study. Review the specific procedure(s) for the sterilization process selected and the methods for controlling and monitoring the process. Verify that the process is controlled and monitored. If review of the Device History Records …
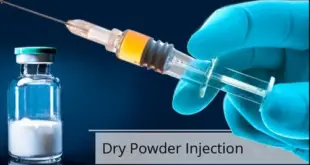
Read More »Dry Powder Injection
Dry Powder Injection Majority of drugs show the problem of poor solubility, whether in the case of their analytical estimations or in the field of liquid dosage forms in the form of solutions. Commonly used organic solvents for spectrophotometric analysis of water insoluble drugs include methanol, ethanol, chloroform, benzene, dichloromethane, …
Read More »Key Point of Dry Powder Injection
The injectable dry filling area is a completely sterile area of the company that is a strictly controlled area. The high-level alertness is mandatory to main the atmospheric condition in the filling area of the dry powder filling area of Injectable. Every step in the production area requires a written …
Read More »BATCH MANUFACTURING RECORD OF DRY INJECTION
BATCH MANUFACTURING RECORD OF DRY INJECTION Table of content Batch Details General Instructions Calculation of API Dispensing of raw material API transfer record Dispensing of primary packing materials Component preparation for sterilization Steam sterilization record Vial de-boxing, Staging, and Inspection record Vial washing record Vial depyrogenation record API Canister weight …
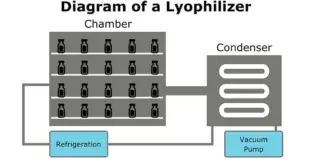
Read More »Lyophilization of Parenteral
Lyophilization of Parenteral Lyophilization or freeze-drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase. The process consists of three separate, unique, and …
Read More »Flow Properties of Powders
Flow Properties of Powders The use of powders in the pharmaceutical industry has generated a variety of methods for characterizing powder flow. Not surprisingly, scores of references appear in the pharmaceutical literature, attempting to correlate the various measures of powder flow to manufacturing properties. The development of such a variety …
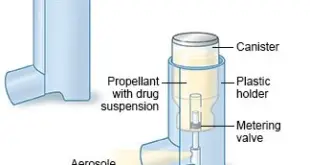
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF METERED – DOSE INHALERS (MDI)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF METERED–DOSE INHALERS (MDI) General .– Manufacture of Metered-Dose-Inhalers shall be done under conditions which shall ensure minimum microbial and particulate contamination. Assurance of the quality of components and the bulk product is very important. Where medicaments are in suspended state, uniformity of suspension shall be established. …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF TOPICAL PRODUCTS
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF TOPICAL PRODUCTS i.e. EXTERNAL PREPARATIONS (CREAMS, OINTMENTS, PASTES, EMULSIONS, LOTIONS, SOLUTIONS, DUSTING POWDERS, AND IDENTICAL PRODUCTS). The entrance to the area where topical products are manufactured shall be through a suitable airlock. Outside the airlock, insectocutors shall be installed. The air to this manufacturing area …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL LIQUIDS (SYRUPS, ELIXIRS, EMULSIONS AND SUSPENSIONS)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL LIQUIDS (SYRUPS, ELIXIRS, EMULSIONS AND SUSPENSIONS) Building And Equipment – The premises and equipment shall be designed, constructed, and maintained to suit the manufacturing of Oral Liquids. The layout and design of the manufacturing area shall strive to minimize the risk of cross-contamination and …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL SOLID DOSAGE FORMS (TABLETS AND CAPSULES)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL SOLID DOSAGE FORMS (TABLETS AND CAPSULES) The processing of dry materials and products creates problems of dust control and cross-contamination. Special attention is, therefore, needed in the design, maintenance, and use of premises and equipment in order to overcome these problems. Wherever required, enclosed …
Read More »Documentation for Manufacture of Sterile products
Documentation for Manufacture of Sterile products The manufacturing records relating to manufacture of sterile products shall indicate the following details: – (1) Serial number of the Batch Manufacturing Record. (2) Name of the product. (3) Reference to Master Formula Record. (4) Batch / Lot number. (5) Batch / Lot size. …
Read More »In-process Checks during Tertiary Packaging Operations
In-process Checks during Tertiary Packaging Operations of cartons/ shrink-wrapped units in one corrugated box / LDPE bag: Count the no. of cartons / shrink wrapped units in one corrugated box / LDPE bag and verify with the Batch Packing Record. Stencil on corrugated boxes: Check the stencil details on the …
Read More »In-process Checks during Secondary Packaging Operations
In-process Checks during Secondary Packaging Operations Performed in In-process quality checks as pre-defined frequency in BPR and to be sorted out the strips/blister with below-mentioned tablet defects before packed in carton or during Packing Broken Tablets Laminated Tablets Chipped Tablets Discoloured Tablets With Black particles Tablets With foreign particle Tablets …
Read More »In-process Checks during Primary Packaging Operations
In-process Checks during Primary Packaging Operations (Blister / Strip Packing) Record the temperature and relative humidity of the Packaging area. Tablets and Capsules: Following defect /Issue to be sorted out before strip/blister sealing Broken Tablets Laminated Tablets Chipped Tablets Discoloured Tablets With Black particles Tablets With foreign particle Tablets Distorted …
Read More »Code of Federal Regulations (21CFR 211)
Organization and Personnel Responsibilities of quality control unit. (a) There shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products, and the authority to review production records to assure …
Read More »Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging
Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging In the pharmaceutical industry, maintaining the integrity and cleanliness of packaging materials is crucial for ensuring the safety and efficacy of drugs. Vial washing machines play a vital role in the production of sterile pharmaceuticals by efficiently cleaning and sanitizing vials before they …
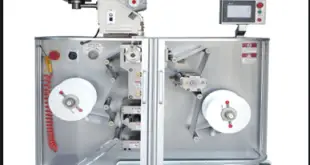
Read More »SOP for operation of Strip Packing Machine
SOP for operation of Strip Packing Machine Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of Strip Packing Machine. Scope This SOP is applicable for the operation of Strip Packing Machine used to pack the Tablets and Capsules into Strips in the formulation plant. Responsibility Production …
Read More »SOP for operation of de-dusting and polishing machine
SOP for operation of de-dusting and polishing machine Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of de-dusting and polishing machine. Scope This SOP is applicable for operation of de-dusting and polishing machine used to polish the Filled Capsules in Capsule Filling Area to the formulation …
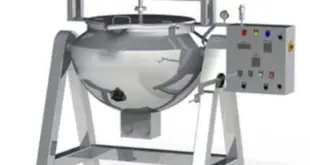
Read More »SOP on operation of Paste Kettle
SOP on operation of Paste Kettle Standard Operating Procedure (SOP) Objective To lay down the procedure for operation of Paste Kettle Scope This SOP is applicable for operation of Paste Kettle in the formulation plant. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ Executive – To …
Read More »