PURIFIED WATER AND WATER FOR INJECTION SYSTEMS
The design, installation ,and operation of systems to produce Purified Water and Water for Injection include similar components, control techniques,and procedures.
The quality attributes of both waters differ only in the presence of a bacterial endotoxin requirement for Water for Injection and in their methods of preparation,at least at the last stage of preparation.
The similarities in the quality attributes provide considerable common ground in the design of water systems to meet either requirement.
The critical difference is the degree of control of the system and the final purification steps needed to ensure bacterial and bacterial endotoxin removal.
Production of pharmaceutical water employs sequential unit operations (processing steps) that address specific water quality attributes and protect the operation of subsequent treatment steps.
The final unit operations used to produce Water for Injection have been limited to distillation and reverse osmosis. Distillation has a long history of reliable performance and can be validated as a unit operation for the production of Water for Injection.
Other technologies such as ultra-filtration may be suitable in the production of Water for Injection,but at this time,experience with this process is not widespread.
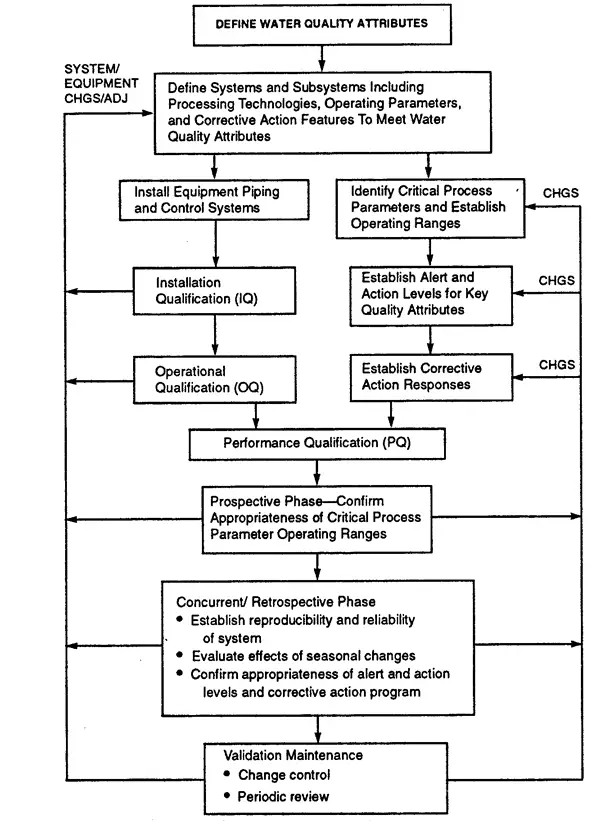
The validation plan should be designed to establish the suitability of the system and to provide a thorough understanding of the purification mechanism, range of operating conditions, required pre-treatment, and most likely mode of failure.
It is also necessary to demonstrate the effectiveness of the monitoring scheme and to establish the requirements for validation maintenance.
Trials conducted in a pilot installation can be valuable in defining the operating parameters and the expected water quality and in identifying failure modes. However,qualification of the specific unit operation can be performed only as part of the validation of the installed operational system.
The selection of specific unit operations and design characteristics for a water system should take into consideration the quality of the feed water, the technology chosen for subsequent processing steps, the extent and complexity of the water distribution system, and the appropriate compendial requirements.
For example, in the design of a system for Water for Injection , the final process (distillation or reverse osmosis) must have effective capability for bacterial endotoxin reduction and must be validated.
The following is a brief description of selected unit operations and the operation and validation concerns associated with them .This review is not comprehensive in that not all unit operations are discussed ,nor are all potential problems addressed.
The purpose is to highlight issues that focus on the design, installation,operation,maintenance,and monitoring parameters that facilitate water system validation.
Filtration technology plays an important role in water systems ,and filtration units are available in a wide range of designs and for various applications. Removal efficiencies differ significantly from coarse filters,such as granular anthracite,quartz,or sand for larger water systems and depth cartridges for smaller water systems,to membrane filters for very small particle control.
Unit and system configurations vary widely in type of filtering media and location in the process.
Granular or cartridge filters are used for pre-filtration. They remove solid contaminants from the water supply and protect downstream system components from contamination that can inhibit equipment performance and shorten its effective life.
Design and operational issues that may affect performance of depth filters include channeling of the filtering media, blockage from silt, microbial growth, and filtering-media loss.
Control measures include pressure and flow monitoring, back-washing,sanitizing,and replacing filtering media. An important design concern is sizing of the filter to prevent channeling or media loss resulting from inappropriate water flow rates.
Activated carbon beds adsorb low-molecular-weight organic material and oxidizing additives, such as chlorine compounds,and remove them from the water.They are used to achieve certain quality attributes and to protect against reaction with downstream stainless steel surfaces,resins,and membranes.
The chief operating concerns regarding activated carbon beds include the propensity to support bacteria growth, the potential for hydraulic channeling, the inability to be regenerated in situ, and the shedding of bacteria, endotoxins,organic chemicals, and fine carbon particles.
Control measures include appropriate high water flow rates, sensitization with hot water or steam, back-washing,testing for adsorption capacity, and frequent replacement of the carbon bed.
Alternative technologies such as chemical additives and regenerable organic scavenging devices can be used in place of activated carbon beds.
Chemical additives are used in water systems to control microorganisms by use of chlorine compounds and ozone, to enhance the removal of suspended solids by use of flocculating agents, to remove chlorine compounds, to adjust pH, and to remove carbonate compounds. Subsequent processing steps are required in order to remove the added chemicals.
Control of additives and subsequent monitoring to ensure removal of additives and of any of their reaction products should be designed into the system and included in the monitoring program.
Organic scavenging devices use macroreticular anion-exchange resins capable of removing organic material and endotoxins from the water. They can be regenerated with appropriate biocidal caustic solutions. Operating concerns are associated with scavenging capacity and shedding of resin fragments. Control measures include testing of effluent, monitoring performance, and using downstream filters to remove resin fines.
Water softeners remove cations such as calcium and magnesium that interfere with the performance of downstream processing equipment such as reverse osmosis membranes, deionization columns,and distillation units.Water softener resin beds are regenerated with sodium chloride solution (brine). Concerns include microorganism proliferation,channeling due to inappropriate water flow rates,organic fouling of resin,fracture of the resin beads,and contamination from the brine solution used for regeneration.
Control measures include recirculation of water during periods of low water use,periodic sanitization of the resin and brine system,use of microbial control devices (e.g.,UVand chlorine),appropriate regeneration frequency,effluent monitoring (hardness),and downstream filtration to remove resin fines.
Deionization (DI), electro-deionization (EDI)and Electrodialysis (EDR) are effective methods of improving the chemical quality attributes of water by removing cations and anions.
DI systems have charged resins that require periodic regeneration with an acid and base. Typically,cationic resins are regenerated with either hydrochloric or sulfuric acid, which replace the captured positive ions with hydrogen ions. Anionic resins are regenerated with sodium or potassium hydroxide, which replace captured negative ions with hydroxide ions. Both regenerant chemicals are biocidal and offer a measure of microbial control.
The system can be designed so that the cation and anion resins are separated or so that they form a mixed bed. Rechargeable resin canisters can also be used for this purpose.
The EDI system uses a combination of mixed resin, selectively permeable membranes, and an electric charge to provide continuous flow (product and waste concentrate)and continuous regeneration. Water enters both the resin section and the waste (concentrate)section. As it passes through the resin, it is deionized to become product water.
The resin acts as a conductor enabling the electrical potential to drive the captured cations and anions through the resin and appropriate membranes for concentration and removal in the waste water stream.The electrical potential also separates the water in the resin (product)section into hydrogen and hydroxide ions.This separation permits continuous regeneration of the resin without the need for regenerant additives.
Electrodialysis (EDR) is a similar process that uses only electricity and selectively permeable membranes to separate, concentrate,and flush the removed ions from the water stream. The process, however,is less efficient than EDI because it contains no resin to enhance ion removal and current flow. Also,EDR units require periodic polarity reversal and flushing to maintain operating performance.
Concerns for all forms of deionization units include microbial and endotoxin control chemical additive effect on resins and membranes and loss, degradation,and fouling of resin. Issues of concern specific to DI units include regeneration frequency, channeling,complete resin separation for mixed bed regeneration and mixing air contamination (mixed beds).
Control measures vary but typically include recirculation loops,microbial control by UV light,conductivity monitoring,resin testing,microporous filtration of mixing air,microbial monitoring,frequent regeneration to minimize and control microorganism growth,sizing the equipment for suitable water flow,and use of elevated temperatures.
Regeneration piping for mixed bed units should be configured to ensure that regeneration chemicals contact all internal surfaces and resins.Rechargeable canisters can be the source of contamination and should be carefully monitored.Full knowledge of previous resin use,minimum storage time between regeneration and use,and appropriate sanitizing procedures are critical factors ensuring proper performance.
Reverse osmosis (RO)units employ a semipermeable membrane and a substantial pressure differential to drive water through the membrane to achieve chemical, microbial,and endotoxin quality improvement. The process streams consist of supply water,product water (permeate),and waste water (reject). Pretreatment and system configuration variations may be necessary,depending on source water to achieve desired performance and reliability.
Concerns associated with the design and operation of ROunits include membrane material sensitivity to bacteria and sanitizing agents,membrane fouling,membrane integrity,seal integrity,and the volume of waste water.Failure of membrane or seal integrity will result in product water contamination.Methods of control consist of suitable pretreatment of the water stream,appropriate membrane material selection,integrity challenges,membrane design such as spiral wound to promote flushing action,periodic sanitization,monitoring of differential pressures,conductivity,microbial levels,and total organic carbon.
The configuration of the ROunit offers control opportunities by expanding the single-pass scheme to parallel-staged,reject-staged,two-pass,and combination designs.An example would be the use of a two-pass design to improve reliability,quality,and efficiency.RO units can be used alone or in combination with DI and EDI units for operational and quality enhancements.
Ultra-filtration is another technology that uses a permeable membrane,but unlike RO it works by mechanical separation rather than osmosis.Because of the filtration ability of the membrane,macromolecular and microbial impurities,such as endotoxins,are reduced.This technology may be appropriate as an intermediate or final purification step.As with RO,successful performance is dependent upon other system unit operations and system configuration.
Issues of concern include compatibility of membrane material with sanitizing agents,membrane integrity,fouling by particles and microorganisms,cartridge contaminant retention,and seal integrity.Control measures include sanitization,designs capable of flushing the membrane surface,integrity challenges,regular cartridge changes,elevated feed water temperature,and monitoring total organic carbon and differential pressure.
Additional flexibility in operation is possible through arrangement of the units,such as parallel or series configuration.Care should be taken to avoid stagnant water conditions that could promote microorganism growth in backup or standby units.
Microbial retentive filters (membrane filters) prevent the passage of microorganisms and very small particles.They are used in tank air and inert gas vents and for filtration of compressed air gases used in the regeneration of mixed-bed deionization units.Areas of concern are blockage of tank vents by condensed water vapor,which can cause mechanical damage to the tank,and concentration of microorganisms on the surface of the membrane filter,creating the potential for contamination of the tank or deionizer contents.
Control measures include the use of hydrophobic filters and heat tracing vent filter housings to prevent vapor condensation.Sterilization of the unit prior to initial use and periodically thereafter or regular filter changes are also recommended control methods.Microbial retentive filters are sometimes incorporated into purification systems or into water distribution piping.
This application should be carefully controlled because,as noted above,these units can become a source for microbial contamination.The potential exists for the release of microorganisms should the membrane filter rupture or as a result of microbial grow-through.Other means of controlling microorganisms and fine particles can be employed in place of membrane filters in the purification and distribution section of water systems.
Filters that are intended to be microretentive should be sanitized and integrity tested prior to initial use and at appropriate intervals thereafter.
Positively charged filter media reduce endotoxin levels by electrostatic attraction and adsorption.Application may be related to the unit operation or distribution system,depending upon the microbial control requirements.Filter media that are microbial retentive require the same concerns and controls as indicated in the previous paragraph.Concerns include flow rate,membrane and seal integrity,and retention capacity,which can be affected by the development of a finite charge potential on the filter.
Control measures include monitoring differential pressure and endotoxin levels,proper sizing,testing membrane integrity,and configuring units in series to control breakthrough.
Distillation units provide chemical and microbial purification via thermal vaporization,mist elimination,and condensing. Avariety of designs are available,including single-effect,multiple-effect,and vapor compression.
The latter two configurations are normally used in larger systems because of their generating capacity and efficiency.Distilled water systems may require less rigorous control of feed water quality than do membrane systems
Areas of concern include carryover of impurities,evaporator flooding,stagnant water,pump and compressor seal design,and conductivity (quality)variations during startup and operation.
Methods of control consist of reliable mist elimination,visual or automated high-water-level indication,use of sanitary pumps and compressors,proper drainage,blow down control,and use of on-line conductivity sensing with automated diversion of unacceptable quality water to the waste stream.
Storage tanks are included in water distribution systems to optimize processing equipment capacity.Storage also allows for routine maintenance while maintaining continuous supply to meet manufacturing needs.
Design and operation considerations are needed to prevent the development of biofilm,to minimize corrosion,to aid in the use of chemical sanitization of the tanks,and to safeguard mechanical integrity.These considerations may include using closed tanks with smooth interiors and the ability to spray the tank head space.This minimizes corrosion and biofilm development and aids in sanitizing thermally or chemically.
Storage tanks require venting to compensate for the dynamics of changing water levels.This can be accomplished with a hydrophobic microbial retentive membrane filter fitted onto an atmospheric vent.
Alternatively,an automatic membrane-filtered compressed gas pressurization and venting system may be used.Rupture disks equipped with a rupture alarm device serve as a further safeguard for the mechanical integrity of the tank.
Distribution configuration should allow for the continuous flow of water in the piping by means of recirculation or should provide for the periodic flushing of the system.Experience has shown that continuously recirculated systems are easier to maintain.
Pumps should be designed to deliver fully turbulent flow conditions to retard the development of biofilms.Components and distribution lines should be sloped and fitted with drain points so that the system can be completely drained.
In distribution systems,where the water is circulated at a high temperature,dead legs and low-flow conditions should be avoided,and valved tie-in points should have length-to-diameter ratios of 6 or less.
In ambient temperature distribution systems,particular care should be exercised to avoid pocket areas and provide for complete drainage. Water exiting from a loop should not be returned to the system.
Distribution design should include the placement of sampling valves in the storage tank and at other locations such as the return line of the recirculating water system.
The primary sampling site for water should be the valves that deliver water to the point of use. Direct connections to processes or auxiliary equipment should be designed to prevent reverse flow into the controlled water system.The distribution system should permit sanitization for microorganism control. The system may be continuously operated at sanitizing conditions or sanitized periodically.
OPERATION,MAINTENANCE,AND CONTROL
A preventive maintenance program should be established to ensure that the water system remains in a state of control. The program should include
(1) procedures for operating the system,
(2)monitoring programs for critical quality attributes and operating conditions,including calibration of critical instruments,
(3)a schedule for periodic sanitization,
(4)preventive maintenance of components,and
(5)control of changes to the mechanical system and to operating conditions.
Operating Procedures—
Procedures for operating the water system and performing routine maintenance and corrective action should be written,and they should also define the point when action is required.The procedures should be well documented,detail the function of each job,assign who is responsible for performing the work,and describe how the job is to be conducted.
Monitoring Program—
Critical quality attributes and operating parameters should be documented and monitored.The program may include a combination of in-line sensors or recorders (e.g.,a conductivity meter and recorder),manual documentation of operational parameters (such as carbon filter pressure drop),and laboratory tests (e.g.,total microbial counts).The frequency of sampling, the requirement for evaluating test results, and the necessity for initiating corrective action should be included.
Sanitization—
Depending on system design and the selected units of operation, routine periodic sanitization may be necessary to maintain the system in a state of microbial control. Technologies for sanitization are described above.
Preventive Maintenance—
Apreventive maintenance program should be in effect.The program should establish what preventive maintenance is to be performed,the frequency of maintenance work,and how the work should be documented.
Change Control—
The mechanical configuration and operating conditions must be controlled.Proposed changes should be evaluated for their effects on the whole system.The need to requalify the system after changes are made should be determined. Following a decision to modify a water system,the affected drawings,manuals,and procedures should be revised.