MANAGEMENT RESPONSIBILITY-THE QUALITY SYSTEMS MODEL
The goal of this section is to describe a model for use in pharmaceutical manufacturing that can help manufacturers comply with CGMP regulations. It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources. However, we believe the long-term benefits of implementing a quality system will outweigh the costs.
This section describes a robust quality systems model that, if properly implemented, can provide the controls to consistently produce a product of acceptable quality. Where applicable, the relationship between elements of this model and CGMP regulations is noted. At the end of each section, a table shows how the specific CGMP regulations correlate to the elements in the quality systems model. As already explained, many of the quality systems elements correlate closely with the CGMP regulations. It is important to emphasize that this guidance is not recommending
new regulatory requirements. The guidance is intended to provide recommendations to manufacturers who are implementing or plan to implement, a quality systems model to help them comply with CGMP regulations. FDA regulatory and inspection coverage will remain focused on the specific CGMP regulations.
The model is described according to four major factors:
- Management Responsibilities
- Resources
- Manufacturing Operations
- Evaluation Activities
In each of the sections that follow, the specific elements of a robust modern quality systems model are described. When elements of the quality systems model correlate with specific CGMP regulations, this correlation is noted.
A. Management Responsibilities
Modern robust quality systems models call for management to play a key role in the design, implementation, and management of the quality system. For example, management is responsible for establishing the quality system structure appropriate for the specific organization.
Management has the ultimate responsibility to provide the leadership needed for the successful functioning of a quality system. This section describes management’s role in developing, implementing, and managing a robust quality system. There is some overlap with the CGMP regulations in this section (see the table at the end of the section).

1. Provide Leadership
In a robust, modern quality system, senior management should demonstrate commitment to developing and maintaining their quality system. Quality system plans should be aligned with a manufacturer’s strategic plans to ensure that the system is part of the manufacturer’s mission and quality strategies. For example, quality systems departments normally have equal standing with other departments within an organization. Quality systems staff are effectively integrated into manufacturing activities and are involved in activities such as nonconformance investigations.
Senior managers set implementation priorities and develop action plans. All levels of management can provide support of the quality system by:
- Actively participating in system design, implementation, and monitoring, including system review (see IV.A.5.)
- Advocating continual improvement of operations of the quality system.
- Committing necessary resources
In a robust quality systems environment, all managers should demonstrate strong and visible support for the quality system and ensure its implementation throughout the organization (e.g., across multiple sites).
All managers should encourage internal communication on quality issues at all levels in the organization. Communication should be ongoing among research and development, regulatory affairs, manufacturing, and QU personnel on issues that affect quality, with management included whenever appropriate.
2. Structure the Organization
When designing a robust quality system, management has the responsibility to structure the organization and ensure that assigned authorities and responsibilities support the production, quality, and management activities needed to produce quality products. Senior managers have the responsibility to ensure that the organization’s structure is documented.
All managers have the responsibility to communicate employee roles, responsibilities, and authorities within the system and ensure that interactions are defined and understood.
An organization also has the responsibility to give the individual who is appointed to manage the quality system the authority to detect problems and implement solutions. Usually, a senior manager administers the quality system and can, thus, ensure that the organization receives prompt feedback on quality issues.
3. Build Your Quality System to Meet Requirements
Implementing a robust quality system can help ensure compliance with CGMP regulations related to drug safety, identity, strength, quality, and purity. Under the quality systems model, the Agency recommends that senior managers ensure that the quality system that is designed and implemented provides clear organizational guidance and facilitates systematic evaluation of issues. For example, according to the model, when documenting the implementation of a quality system, the following should be addressed:
- The scope of the quality system, including any outsourcing (see IV.B.4.)
- The quality standard will be followed.
- The manufacturer’s policies to implement the quality systems criteria and the
supporting objectives (see IV.A.4.) - The procedures needed to establish and maintain the quality system
It is recommended under a modern quality systems approach that a formal process is established to change procedures in a controlled manner. It is also recommended that when operating under a quality system, manufacturers develop and document control procedures to complete, secure, protect, and archive records, including data, which provide evidence of operational and quality system activities. This approach is consistent with the CGMP regulations, which require manufacturers to establish and follow scientifically sound and appropriate written controls for specifications, plans, and procedures that direct operational and quality system activities and to
ensure that these directives are accurate, appropriately reviewed and approved, and available for use (see the CGMPs at § 211.22 (c) and (d)).
4. Establish Policies, Objectives, and Plans
Policies, objectives, and plans under a modern quality system provide the means by which senior managers articulate their vision of and commitment to quality to all levels of the organization.
Under a quality system, senior management should incorporate a strong commitment to quality into the organizational mission. Senior managers should develop an organizational quality policy that aligns with this mission; commit to meeting requirements and improving the quality system, and propose objectives to fulfill the quality policy. Under a quality system, make the policy-relevant, it must be communicated to and understood by, personnel and contractors (if applicable) and revised, as needed.
Managers operating within a quality system should define the quality objectives identified for implementing the quality policy. Senior management should ensure that the quality objectives are created at the top level of the organization (and other levels as needed) through a formal quality planning process. Objectives are typically aligned with the manufacturer’s strategic plans.
A quality system seeks to ensure that managers support the objectives with necessary resources and have measurable goals that are monitored regularly.
Under a quality systems approach, managers would use quality planning to identify and allocate resources and define methods to achieve quality objectives. Quality system plans should be documented and communicated to personnel to ensure awareness of how their operational activities are aligned with strategic and quality goals.
5. Review the System
System review is a key component in any robust quality system to ensure its continuing suitability, adequacy, and effectiveness. Under a quality system, senior managers should conduct reviews of the quality system’s performance according to a planned schedule. Such a review typically includes assessments of the process, product, and customer needs (in this section, customer is defined as the recipient of the product and the product is the goods or services provided). Under a quality systems approach, a review should consider at least the following:
- The appropriateness of the quality policy and objectives
- The results of audits and other assessments
- Customer feedback, including complaints
- The analysis of data trending results
- The status of actions to prevent a potential problem or a recurrence
- Any follow-up actions from previous management reviews
- Any changes in business practices or environment that may affect the quality system(such as the volume or type of operations)
- Product characteristics meeting the customer’s needs
When developing and implementing new quality systems, reviews should take place more frequently than when the system has matured. Outside of scheduled reviews, the quality system should typically be included as a standing agenda item in general management meetings. In addition, a periodic review performed by a qualified source, external to the organization, may also be useful in assessing the suitability and effectiveness of the system.
Review outcomes typically include:
- Improvements to the quality system and related quality processes
- Improvements to manufacturing processes and products
- Realignment of resources
Under a quality system, the results of a management review would typically be recorded.
Planned actions should be implemented using effective corrective and preventive action and change control procedures.
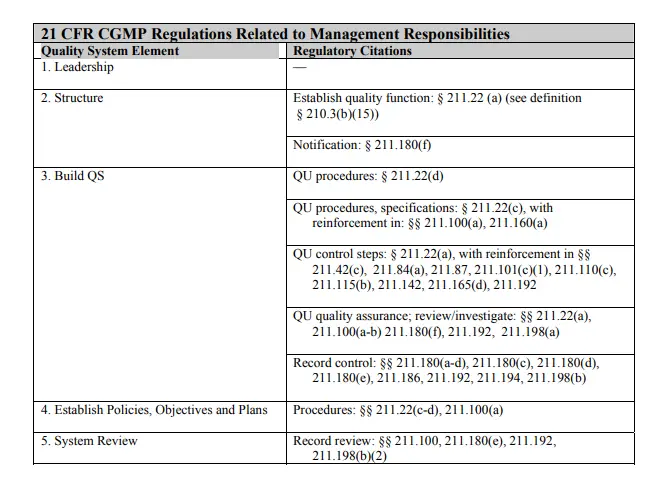
The following table shows how the CGMP regulations correlate to specific elements in the quality systems model for this section. Manufacturers should always refer to specific regulations to make sure they are in compliance.
MANUFACTURING-THE QUALITY SYSTEMS MODEL
RESOURCES-THE QUALITY SYSTEMS MODEL
CGMPS AND THE CONCEPTS OF MODERN QUALITY SYSTEMS


