Human Errors
- Understanding Human Errors
- Regulatory expectations
- The current way of handling human errors
- Types of human errors
- Investigation of human errors
- CAPAs
- Case study
Human Error Is The Leading Cause Of GMP Deviations – 25-60% of the deviations / Incidents in the companies are caused by human errors
Human Errors Regulatory expectations {(as per PICS – PE-006-14 Part – I,1 July 2018 (xiv)}
- An appropriate level of root cause analysis should be applied during the investigation of deviations, suspected product defects, and other problems.
- This can be determined using Quality Risk Management principles. In cases where the true root cause(s) of the issue cannot be determined, consideration should be given to identifying the most likely root cause(s) and to addressing those.
- Where human error is suspected or identified as the cause, this should be justified having taken care to ensure that process, procedural or system-based errors or problems have not been overlooked, if present.
- Appropriate corrective actions and/or preventive actions (CAPAs) should be identified and taken in response to investigations.
- The effectiveness of such actions should be monitored and assessed, in line with Quality Risk Management principles;
In summary,
1. Small quantity of deviations to result from human error
2. Classify it as Human error as a last resort.
3. Eliminated any possible process, environment, procedural or system-based issues
Warning Letter / FDA 483
1. Foreign matter was identified as a known process-related defect, yet no specific root cause for the particulate was identified. And the most likely root cause of failure to identify the critical/major defects during 100% visual inspection was identified as human error.
2. High percentage rate of invalidated OOS (77%) test results without appropriate investigation was identified as contributing mainly because of human error, instrument/column error, and method error.
3. Multiple LI investigations lacked scientific rationale for root cause determination. Probable root cause were attributed to contamination and analyst error
4. CAPAs have often been limited to retraining analysts. Improvements in analytical methods and equipment were not generally implemented to enhance the robustness and prevent error
Human Errors – Current Ways
• Blame, Blame and Blame!
- Active blaming
- Passive blaming
Human Errors – Current CAPAs
• Training / Re-training
• Display notification
• Take action on the employee
• One point lesson
• Revise SOP
| Human errors | Unintended Actions | Action not as planned (Error) Attention gap |
Slip of action |
| Lapse of memory | |||
| Action as planned (Mistake) |
Rule-based mistake Understanding Gap |
||
| Knowledge-based mistake Skill gap | |||
| Intended Actions (Behavioral Gap) |
Routine | – | |
| Situational | – | ||
| Exceptional | – |
Human Errors
| Attention Gap | Understanding Gap | Proficiency Gap | Behavioral Gap |
| Examples | Examples | Examples | Examples |
| 1.Memory gap / forgetfulness 2. Lazy 3. Attention toward work 4. Omission of action 5. Absent-mindedness |
1. Learning gap 2. Decision error 3.Procedural / SOP 4. Complex system 5. Communication gap 6. Judgement error |
1. Inadequate knowledge 2. Skill / Analytical ability 3. Concept application error |
1. Work environment
Attitude 3. Culture 4. Physical / Mental limitation 5. Intentional errors |
| Probable causes • Clear Job responsibilities • Infrastructure • Fatigue • Work pressure/overload • Work allocation |
Probable causes • Training • SOP / Instructions • Communication mechanism • Overconfident |
Probable causes • Lack of knowledge • Decision error • Suitability for the role • Complex systems / procedure |
Probable causes • Incorrect R&R, • Collaboration • Leadership focus • Metrics • Habitual |
Most common human errors in Pharma
Manufacturing
1. Documentation
2. Labeling
3. Line clearance
4. Schedule misses
Quality Assurance
1. Document review misses
2. Retain sample review
Laboratory / OOS
1. Solution preparation
2. Dilution
3. Weighing
4. Documentation
Engineering
1. PM / Calibration schedule misses
2. Documentation
Do all human errors require investigation?
Does all human errors be investigated and CAPA implemented?
E.g. Skips, Lapses
Risk tools
• Severity: Safety, Quality
• Detectability: Already checks available to detect it
• Frequency: No. of occurrences
Categorization: Human Factor
Physical
Physical Capability
– Vision / Hearing / Sensory
– Disabilities
– Restricted body movements
– Difficult body positions
Physical Condition
– Injury
– Illness
– Insufficient Rest
– Oxygen deficiency
Mental
Mental State
– Memory
– Reaction time
– Medication
Mental Stress
– Frustration
– Conflicting communications
– Too many problems
Behavior
– Shortcuts
– Improper reward
– Avoids discomfort
– Relax attitude
Skills
– Wrong skills
– Insufficient training / OJT
– Improper assessment
Knowledge Transfer
Clear and concise operating instructions
– Improper risk assessments and controls
Engineering Design
Design of area / equipment / system
– Standards
– Ergonomics
– Change management of engg changes
Work Planning
– Work allocation
– Output orientation (e.g. In-sufficient PM)
Policies
– Induction
– R&R
– Risk assessments: Acceptable risk ratings
Management / Supervision
– Assignment of roles
– Delegation
– Standard work
– Performance dialogues
Communications
– No clear communication
– Focus on speed
Human Errors – Investigation
Important points for human error investigations
• Pre-defined Interview checklist.
• Photographic evidence
• Approved hypothesis plan (Wherever required)
• Spot verification (Gemba walks)
• Data analysis based on system, person, area, process, system etc
Human Errors Summary
1. Human errors do happen
2. Categorize it as Human error after all possible causes have been negated.
3. Small quantity of deviations to result from human error
4. Investigation should be thorough to ensure that cause is identified.
5. Eliminated any possible process, environment, procedural, or system-based issues
6. Classify human errors in Attention gap, understanding gap, skill gap, and behavioral gap
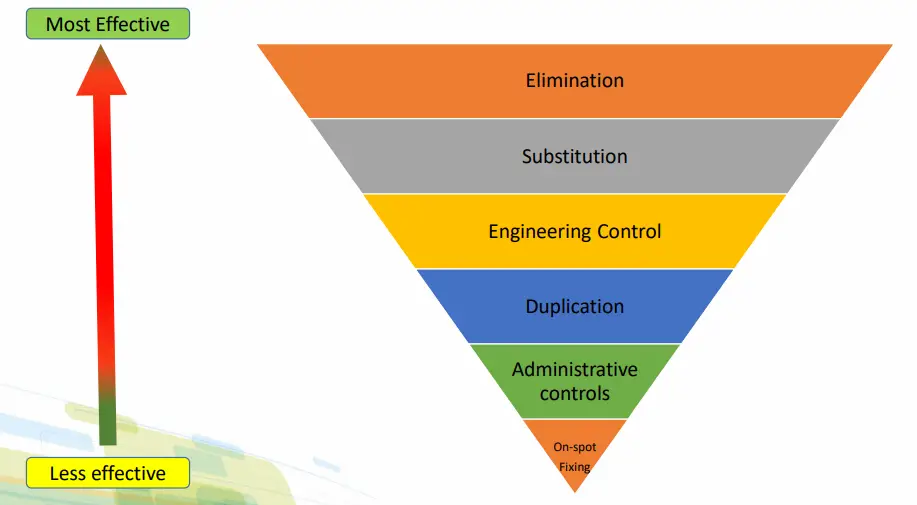
7. Take appropriate actions based on the causes
8. Look for error proofing instead of blame, duplication, etc.