Dispensing Booth
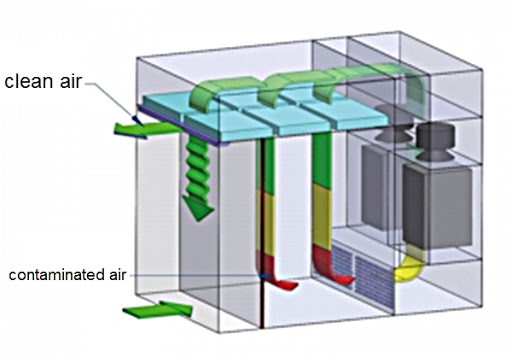
A dispensing booth is a kind of laminar flow filtration cabin that is employed in dispensing, sampling, and weighing powders and chemicals in the pharma, biotech, and chemical industries. Such units work on reverse laminar flow working principle and protect operators, products/samples, and the surrounding environment.
The dispensing booth is usually made of stainless steel and equipped with three-stage filtration (10µ Pre-filter, 310µ Intermediate, and 0.310µ HEPA Filter) with the help of a motor blower assembly. To measure the pressure of the HEPA filter, Magnehelic gauges are fitted. Pre-filters are washable.
Also known as sampling booths or weighing booths, these units are made requirements of pharmaceutical and biotech companies in mind. Before dispatch, each unit is tested in-house at various parameters.
- Imported Mini pleat HEPA Filter with hot melt technology, which confirms to EU 13 Grade, with an efficiency rating better than 99.99% for 0.3μ at the supply position
- Prefilter confirms to EU 4 Grade, with efficiency of 90% down to 10µ. These Filters are made from micro-fibre-glass media and are inherently washable.
- Intermediate filter which confirms to EU 7 Grade, with efficiency of 95% down to 3µ. These Filters are made from micro-fibre-glass media and are inherently washable.
- The motor Blower provided is statically and dynamically balanced, Supply of sufficient capacity and static pressure is used to take care of airflow requirements for the entire life of HEPA. The blower is high-performance, noise-abated, light weighted statically, and dynamically balanced
- SS 304 Double Walled Side Panels
- Differential Pressure Gauge HEPA filter. To monitor pressure drop across the PRE Filter concerning ambient
- Electrical control panel mounted on the side panel of the unit
- Tube Light
- ON/OFF Switches for motor
- DOP Test port at upstream of HEPA filter
Performance Qualification Protocol of Dispensing Booth
 Loading...
Loading...