MANUFACTURING-THE QUALITY SYSTEMS MODEL
Significant overlap exists between the elements of a quality system and the CGMP regulation requirements for manufacturing operations. It is important to emphasize again that FDA’s enforcement programs and inspectional coverage remain based on the CGMP regulations. When quality system elements in this section do not correlate to the CGMP regulations, the guidance makes recommendations to help facilitate compliance with the CGMP regulations. The language in this section has been tailored to the pharmaceutical manufacturing environment.
1. Design, Develop, and Document Products and Processes
In a modern quality systems manufacturing environment, the significant characteristics of the product being manufactured should be defined from design to delivery, and control should be exercised over all changes. In addition, quality and manufacturing processes and procedures —and changes to them — must be defined, approved, and controlled (§ 211.100). It is important to establish responsibility for designing or changing products. Documenting processes, associated controls, and changes to these processes will help ensure that sources of variability are identified.
Documentation includes:
- Resources and facilities used
- Procedures to carry out the process
- Identification of the process owner who will maintain and update the process as needed
- Identification and control of important variables
- Quality control measures, necessary data collection, monitoring, and appropriate controls for the product and process
- Any validation activities, including operating ranges and acceptance criteria
- Effects on related process, functions, or personnel
As discussed under section IV.A., above, the model calls for managers to ensure that product specifications and process parameters are determined by the appropriate technical experts (e.g., engineers, and development scientists). In the pharmaceutical environment, experts would have an understanding of pharmaceutical science, equipment, facilities, and process types and of how variations in materials and processes can ultimately affect the finished product.
Packaging and labeling controls, critical stages in the pharmaceutical manufacturing process, are not specifically addressed in quality systems models. However, the Agency recommends that manufacturers always refer to the packaging and labeling control regulations at § 211 Subpart G.
In addition — and this is consistent with modern quality systems — FDA recommends that, as part of the design process, before commercial production, the controls for all processes within the packaging and labeling system be planned and documented in written procedures. The procedures should outline quality control activities and the responsible positions. Specifications and controls for the packaging and labeling materials should also be determined before commercial production. Distinct labels with discriminating features for different products, such as a product marketed with different strengths, should be included to prevent mislabeling and resulting recalls.
2. Examine Inputs
In a modern quality systems model, the term input includes any material that goes into a final product, no matter whether the material is purchased by the manufacturer or produced by the manufacturer for the purpose of processing. Materials can include items such as components (e.g., ingredients, process water, and gas), containers, and closures. A robust quality system will ensure that all inputs to the manufacturing process are reliable because quality controls will have been established for the receipt, production, storage, and use of all inputs.
The CGMP regulations require either testing or use of a certificate of analysis (COA) plus an identity analysis (§ 211.84) for the release of materials for manufacturing. In the preamble to the CGMP regulations, these requirements were explicitly interpreted.12 The preamble states that reliability can be validated by conducting tests or examinations and comparing the results to the supplier’s COA. Sufficient initial tests should be done to establish reliability and to determine a schedule for periodic reassessment. As an essential element of purchasing controls, it is
recommended that data trends for acceptance and rejection of materials be analyzed for information on supplier performance.
The quality systems approach also calls for periodic auditing of suppliers based on risk assessment. During the audit, a manufacturer can observe the testing or examinations conducted by the supplier to help determine the reliability of the supplier’s COA. An audit should also include a systematic examination of the supplier’s quality system to ensure that reliability is maintained. It is recommended that a combination approach be used (i.e., verify the suppliers’
COA through analysis and audits of the supplier). Under a quality systems approach, if full analytical testing is not done, the audit should cover the supplier’s analysis (i.e., a specific identity test is still required under § 211.84(d)(2)).
Under a quality systems approach, procedures should be established to verify that materials are from qualified sources (for application and licensed products, certain sources are specified in the submissions). Procedures should also be established to encompass the acceptance, use, or the rejection and disposition of materials produced by the facility (e.g., purified water). Systems that produce these in-house materials should be designed, maintained, qualified, and validated where appropriate to ensure that the materials meet their acceptance criteria.
In addition, it is recommended that changes to materials (e.g., specification, supplier, or materials handling) be implemented through a change control system (certain changes require review and approval by the QU (§ 211.100(a)). It is also important to have a system in place to respond to changes in materials from suppliers so that necessary adjustments to the process can be made and unintended consequences avoided.
3. Perform and Monitor Operations
An important purpose of implementing a quality systems approach is to enable a manufacturer to more efficiently and effectively validate, perform, and monitor operations (§ 211.100(a)) and ensure that the controls are scientifically sound and appropriate. The goal of establishing, adhering to, measuring, and documenting specifications and process parameters is to objectively assess whether an operation is meeting its design and product performance objectives. In a robust quality system, production and process controls should be designed to ensure that the finished products have the identity, strength, quality, and purity they purport or are represented
to possess (see, e.g., § 211.100(a)).
In a modern quality system, a design concept established during product development typically matures into a commercial design after process experimentation and progressive modification.
Risk management can help identify areas of process weakness or higher risk and factors that can influence critical quality attributes that should receive increased scrutiny. The FDA recommends that scale-up studies be used to help demonstrate that a fundamentally sound design has been fully realized. A sufficiently robust manufacturing process should be in place prior to commercial production. With proper design (see IV.C.1.) and reliable mechanisms to transfer process knowledge from development to commercial production, a manufacturer should be able to validate the manufacturing process.14 Conformance batches provide initial proof that the design of the process produces the intended product quality. Sufficient testing data will provide essential information on the performance of the new process, as well as a mechanism for continual improvement. Modern equipment with the potential for continual monitoring and control can further enhance this knowledge base. Although initial commercial batches can provide evidence to support the validity and consistency of the process,15 the entire product life cycle should be addressed by the establishment of continual improvement mechanisms in the quality system.
Thus, in accordance with the quality systems approach, process validation is not a one-time event, but an activity that continues throughout a product’s life.
As experience is gained in commercial production, opportunities for process improvements may become evident. (CGMP regulations § 211.180 require the review and evaluation of records to determine the need for any change. These records contain data and information from production that provide insight into the product’s state of control. Change control systems should provide a dependable mechanism for prompt implementation of technically sound manufacturing improvements.)
Under a quality system, written procedures are followed and deviations from them are justified and documented (CGMP requires this; see § 211.100(b)) to ensure that the manufacturer can trace the history of the product, as appropriate, concerning personnel, materials, equipment, and chronology and that processes for product release are complete and recorded.
Both the CGMP regulations (§ 211.110) and quality systems models call for the monitoring of critical processes that may be responsible for causing variability during production. For example:
- Process steps must be verified by a second person (§ 211.188). Process steps can also be performed using a validated computer system. Batch production records must be prepared contemporaneously with each phase of production (§ 211.100(b)). Although time limits for production can be established when they are important to the quality of the finished product (CGMP addresses this; see § 211.111), the manufacturer should have the
ability to establish production controls using in-process parameters that are based on desired process endpoints measured using real-time testing or monitoring apparatus (e.g., blend until mixed vs. blend for 10 minutes). - Procedures must be in place to prevent objectionable microorganisms in finished products not required to be sterile and to prevent microbial contamination of finished products purported to be sterile. Sterilization processes must be validated for sterile drugs (§ 211.113(b)).
Manufacturing processes must consistently meet their parameters, and in-process materials must meet acceptance criteria or limits (§ 211.110(b) and (c)) so that, ultimately, finished pharmaceutical products will meet their acceptance criteria. Under a quality system, selected data are used to evaluate the quality of a process or product. In addition, data collection can provide a means to encourage and analyze potential suggestions for improvement. A quality systems approach calls for the manufacturer to develop procedures that monitor, measure, and analyze the operations (including analytical methods and/or statistical techniques).
Monitoring of the process is important due to the limitations of testing. Knowledge continues to accumulate from development through the entire commercial life of a product. Significant unanticipated variables should be detected by a well-managed quality system and adjustments implemented. Procedures should be revisited as needed to refine the operational design based on new knowledge. Process understanding increases with experience and helps identify when the change will lead to continual improvement. When implementing data collection procedures, consider the following:
- Are data collection methods documented?
- When in the product life cycle will the data be collected?
- How and to whom will measurement and monitoring activities be assigned?
- When should analysis and evaluation (e.g. trending) of laboratory data be performed?(see IV.D.1).
- What records should be collected?
A modern quality system approach indicates that change control is warranted when data analysis or other information reveals an area for improvement. Changes to an established process must be controlled and documented to ensure that desired attributes for the finished product will be met (§ 211.100(a)).
Change control with regard to pharmaceuticals is addressed in more detail in the CGMP regulations. When developing a process change, it is important to keep the process design and scientific knowledge of the product in mind. If major design issues are encountered through process experience, a firm may want to revisit the adequacy of the design of the manufacturing facility (§ 211.42), the design of the manufacturing equipment (§ 211.63), the design of the production and control procedures (§ 211.100), or the design of laboratory controls (§ 211.160).
When implementing a change, its effect should be determined by monitoring and evaluating those specific elements that may be affected based on an understanding of the process. This approach allows the steps taken to implement a change and the effects of the change on the process to be considered systematically.
The application of risk analysis may facilitate evaluating the potential effect of the change. Evaluating the effects of a change can entail additional tests or examinations of subsequent batches (e.g., additional in-process testing or additional stability studies). The quality system elements identified in this guidance, if implemented and maintained,
will help a manufacturer manage change and implement continual improvement in manufacturing.
Under a quality systems approach, procedures should be in place to ensure the accuracy of test results. Test results that are out of specification may be due to testing problems or manufacturing problems and should be investigated. Any invalidation of a test result should be scientifically sound and justified.
To maintain quality, the Agency recommends that prior to completion of manufacturing, the manufacturer should consider storage and shipment requirements to meet special handling needs (in the case of pharmaceuticals, one example might be refrigeration).
Under a quality system, trends should be continually identified and evaluated. One way of accomplishing this is the use of statistical process control. The information from trend analyses can be used to continually monitor quality, identify potential variances before they become problems, bolster data already collected for the annual review, and facilitate improvement throughout the product life cycle. Process capability assessment can serve as a basis for
determining the need for changes that can result in process improvements and efficiency (see IV.D.1.).
4. Address Nonconformities
A key component in any quality system is handling nonconformities and/or deviations. The investigation, conclusion, and follow-up must be documented (§ 211.192). To ensure that a product conforms to requirements and expectations, it is important to measure the process and the product attributes (e.g., specified control parameters, strength) as planned. Discrepancies may be detected during any stage of the process or during quality control activities. Not all discrepancies will result in product defects; however, it is important to document and handle discrepancies appropriately. A discrepancy investigation process is critical when a discrepancy is found that affects product quality (CGMP also requires this; see § 211.192).
In a quality system, it is important to develop and document procedures that define who is responsible for halting and resuming operations, recording non-conformities, investigating discrepancies, and taking remedial action. Under a quality system, if a product or process does not meet requirements, it is essential to identify and/or segregate the product so that it is not distributed to the customer. Remedial action can include any of the following:
- Correct the non-conformity
- With proper authorization, allow the product to proceed with the justification of the conclusions regarding the problem’s impact.
- Use the product for another application where the deficiency does not affect the quality of the product
- Reject the product
The corrected product or process should also be re-examined for conformance and assessed for the significance of the non-conformity (see, e.g., § 211.115). If the non-conformity is significant, based on consequences to process control, process efficiency, product quality, safety, efficacy, and product availability, it is important to evaluate how to prevent recurrence (see IV.D.4.). If an individual product does not meet requirements has been released, the product can be recalled.18 Customer complaints must be reviewed and then investigated if a discrepancy
is identified (§ 211.198).
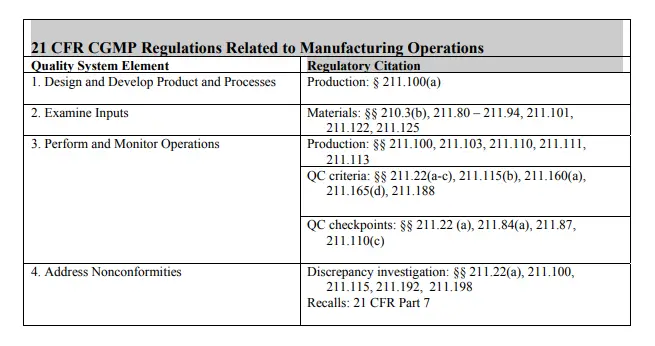
The following table shows how the CGMP regulations correlate to specific elements in the quality systems model. Manufacturers should always refer to specific regulations to ensure that they are complying with all regulations.
RESOURCES-THE QUALITY SYSTEMS MODEL