HAZARD ANALYSIS OF CRITICAL CONTROL POINT (HACCP)
TABLE OF CONTENTS
HAZARD ANALYSIS OF CRITICAL CONTROL POINT
1. Declaration.
Quality Policy
Quality Objectives
Company Profile
Layout of Plant
Organization Chart
HACCP
- Introduction
- HACCP Prerequisite Programs
- Seven Principles of HACCP
- Hazards
HACCP Scope
HACCP Team Responsibility
Hazard Analysis
- Introduction
- Hazard Analysis for processing steps
- Hazard Analysis Worksheet for RM
- Hazard Analysis Work Sheet for Packing Material
- Hazard Analysis Work Sheet for Additives
Product Profile
CAPSULES
- Production flow chart of capsules
- Material dispensing process
- Bulk preparation and capsule filling
- Filled capsules inspection, labeling & packing process
Processing Steps of Capsules (Process Description)
On-Site verification of process Flow for Capsule
Hazard Analysis Worksheet for Capsule
CCP decision Tree
CCP Decision Tree Analysis
Justification of CCP/ Critical Limits
Justification of CQP
HACCP (CCP) Plan Form for Moringo Capsule
Predetermined Corrective /Preventive Action
Sampling Plan for the Establishment
Water management system
Effluent Treatment Plant/ Solid Waste Management
Pest / Rodent Control
- Introduction
Traceability/Re-Call Procedures/Shelf Life Study
Verification Procedure
Training
- Training Objective
- Awareness, and Responsibilities
- Training Programme
Buyers Complaint Procedures
Policies
- Glass /Plastic Policy
- Metal Policy
1. PRP, that manages the basic condition and activities.
2. OPRP, that manages those control measures that the hazard analysis identifies as necessary to control identified hazards to acceptable levels and which are not controlled by HACCP PLAN.
3. HACCP PLAN, that manages those control measures that the hazard analysis identifies as necessary to control identified hazard to acceptable levels and which are applied at CCP.
1. Declaration
I am pleased to declare that this HACCP manual is authentic, original and the procedure and activities enumerated in this manual are true & correct.
This manual complies with HACCP / GMP regulation (21 CFR, part110) and / or codex alimentary guidelines on GMP (EC directives; 91/493/EEC and 94/356/EC). The manual further covers the procedure of SOP, SSOP & quality control programs,s, etc.
This manual also covers the requirement of the EIC (Export Inspection Council) Govt. of India.
This is the first manual and procedure outline in this manual are mandatory and shall be followed by all the employees of M/s Sano Cito Therapeutics Inc
This shall be valid for a period of 12 months from _____________to______________.
Issued by
Warning: No part of this document may be reproduced in any form without the written authorization of the Company Name_______________________. All inquiries regarding this manual shall be directed to the “Management representative” for its administration.
2. Quality Policy
The company is committed to guarantee customer satisfaction for every single use of the product by providing quality, legal and safe Nutraceuticals by timely processing and in-time delivery which is achieved through effective teamwork and continual improvement.
3. Quality Objectives
The quality objectives of the products are as followed,
- To ensure optimal health, Hygiene, and cleanliness, a necessary temperature-controlled environment is maintained in the entire manufacturing process.
- Maintain Humidity control and pathogen-free atmosphere
- Yield Objectives:
| Product | Yield | |
| 1 | Capsules | |
| 2 | Tablets | |
| 3 | Sachet |
The above quality objectives are monitored and maintained quarterly by General Manager.
The Yield in percentage may be varying with various products, grades, and size,s and packing style.
Signature of Management Representative
Food Safety Objectives
The food safety objectives are as follows:
- To achieve the total hours of food safety training to a minimum of 12 hours per year per employee.
- Not more than 4 delayed orders for food safety reasons in the year.
- To ensure that the incidence of end-customer complaints does not occur in more than 1000 PPM of units produced.
- To ensure 100% investigation of customer complaints & written response within 7days.
- Not more than 0.1% of packed products are returned because of failing packaging per month.
- The above food safety objectives are monitored and maintained quarterly by Directors.
Signature of Management Representative.
5. Company Profile
Brief Introduction of Ayurvedic Company
We appreciate exploring the opportunity of manufacturing some Ayurvedic/nutraceutical products in our newly commissioned unit and building a cornerstone in the Industry.
6. Layout of Plant
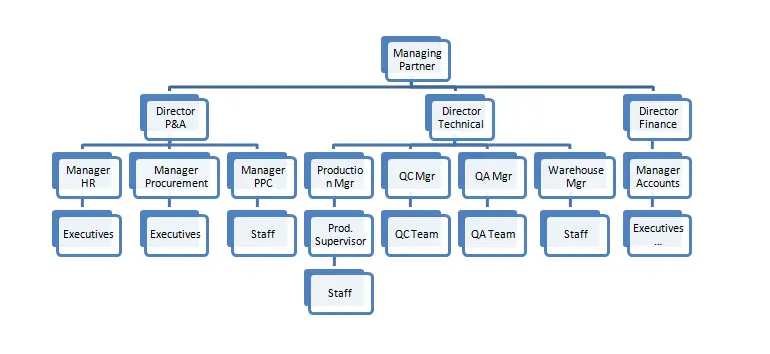
7. Organisation Chart
8. HACCP
I. Introduction
HACCP is an acronym that stands for Hazard Analysis and Critical Control Point. HACCP is a preventive system of hazard control. Food processors use it to ensure safer food for consumers. The HACCP system is designed to identify hazards, establish critical control points and control measures, and monitor these controls. Hazards can be harmful biological, chemical, and physical contaminants.
II. HACCP Prerequisite Programs
In order for HACCP to be successful, it must be built upon a firm foundation of
- (i) compliance with current Good Manufacturing Practices (GMPs) and acceptable Sanitation Standard Operating Procedures (SSOPs),
- (ii) Trained company personnel, and
- (iii) Management commitment at the highest level.
III. Seven Principles of HACCP
There are seven basic principles of HACCP as under
Principle 1: Hazards Analysis: Conduct a hazard analysis. Prepare a list of steps in the process where significant hazards could occur and describe the preventive measures.
Hazard: A biological, Chemical or Physical property that may cause a food to be unsafe for consumption.
Principle 2: Critical Control Point Identification. Identify the critical control points in the process
Critical Control Point: A point, step or procedure at which control can be applied and a food safety hazard can be prevented eliminated or reduced to acceptable levels.
Principle 3: Establish Critical Limits, Establish Critical Limits for preventive measures associated with each identified critical control point.
Critical Limit: It means a creation that must be met for each preventive measure associated with a critical control point.
Principle 4: Establish Monitoring Requirements: Establish Critical Control Point monitoring requirements. Establish procedures for using the results of monitoring to adjust the process and maintain control.
Monitor: To conduct a planned sequence of observations or measurements to assess whether a critical control point is under control and to produce an accurate record for future use in verification.
Principle 5: Establish Corrective Actions: Establish corrective actions to be taken when monitoring indicates that there is a deviation from an established critical limit.
Corrective Action: Procedures followed when a deviation from a critical limit occurs at a critical control point.
Principle 6: Establish Verification Procedures: Establish procedures for verification that the HACCP system is working correctly.
Verification: The use of methods, procedures or tests, in addition to those used in monitoring, that determine if the HACCP system is in compliance with the HACCP plan and / or whether the plan needs modification and revalidation.
Principle 7: Establish Record-Keeping Procedures
Establish effective record-keeping procedures that document the HACCP system.
During the hazard analysis and subsequent HACCP plan design and application, consideration must be given to a number of factors. These include; the impact of raw materials, ingredients, and good manufacturing practices, the role of manufacturing processes to control hazards; the likely end-use of the product; consumer populations at risk; and data relative to food safety.
The intent of the HACCP system is to focus control at the critical control points for minimizing the greatest risks to product safety. Redesign of the operation should be considered if a significant hazard is identified but no critical control points are found.
Flexibility within the context of the operation should be maintained in the application of HACCP to each specific operation. The HACCP plan should be reviewed and necessary changes made to it when a modification is made to the product or a manufacturing step, which changes the significance of a hazard(s) or alters the control or monitoring activities of a Critical Control Point.
IV. Hazards
A hazard is a biological, chemical, or physical property that may cause a food to be unsafe for consumption. To perform a hazard analysis for the development of a HACCP plan, knowledge of potential hazards is very essential. The HACCP plan is designed to control all “significant” food safety hazards. Food safety hazards are categorized into three classes they are Biological, Chemical, and Physical. It is important to understand that, for the purpose of HACCP, hazards only refer to the conditions or contaminants in food that can cause illness or injury to people. Many conditions are highly undesirable in food, such as the presence of insects, hair, filth, or spoilage. Economic fraud and violations of regulatory food standards are equally undesirable. All of these defects must be controlled in food processing.
1. Chemical Hazards
Chemical contamination can happen at any stage in food production and processing, Chemicals are not hazardous if properly used or controlled. Potential risks to consumers increases when chemicals are not controlled or the recommended treatment rates are exceeded. The presence of a chemical may not always represent a hazard. The amount of the chemical may determine whether it is a hazard or not. Some may require exposure over prolonged periods to have a toxic effect. Regulatory limits are set for some of those contaminants.
2. Biological Hazards
Food-borne biological hazards include bacterial organisms. Bacterial Pathogens comprise the majority of food-borne diseases. Many of these pathogens occur naturally in the environment. A certain level of pathogens can be expected with some raw materials. Temperature abuse i.e. holding high/low temperature can significantly multiply numbers or activate pathogens.
3. Physical Hazards
Physical hazards may include potentially harmful extraneous matter that includes dirt and filth.
During blending operations and with equipment that has part that can break or tear off, such as metal parts.
4. Economical Hazards
Another significant hazard can be effect of the commerciality of the product due to mistake of product description i.e. non-conformity of product declaration vis-a-vis, weight count, etc. This hazard can be the result of the workmanship during course of production and can be controlled by proper supervision.
9. HACCP Scope
A view of improving its overall efficiency and effectiveness has focused on the custom and process approach and has adopted the Hazard Analysis Critical Control Point system.
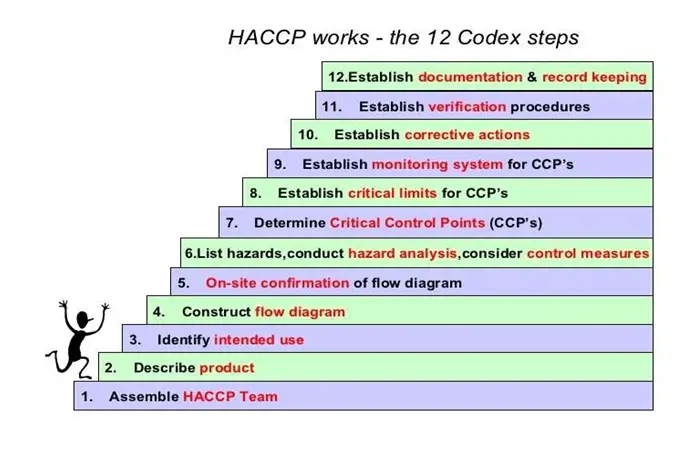
Purpose: This manual covers in detail from raw material receiving to still shipment. The Hazard Analysis and Hazard Plan for all products processed within the company with a view to ensuring food safety of the product especially the following are covered as per Codex Alimentary Steps.
10. Steps of HACCP:
11. HACCP Team Responsibility
11.1 HACCP / FOOD SAFETY
The HACCP team has been formed and approved by the Managing Partner of the company, keeping in mind the management’s commitment to implement the quality system in totally. The HACCP team leader is appointed by the managing partner.
Managing Director is the chief authority for the welfare official staff & workers as well as an administrative, financial sanction for implementing the quality system of our company. The Quality Assurance In-charge is the focal point of the HACCP team. In the absence of the HACCP team leader, the Quality Assurance Executive will have the team leader responsibilities and the same shall be communicated to the HACCP team time to time. Other member in the team is the department’s head of the following areas.
11.2 HACCP Team Competency and Training
Team leader / Quality Assurance In charge provides training to the new members of the HACCP team on HACCP & site procedures on a requirement basis based on the review of monthly team meetings. The team leaders shall provide training to the members and other staff to increase their competency.
11.3 HACCP / Food Safety Team
As it is a collective effort or teamwork to produce a good product, the following are the responsibilities so the team members to do so.
| Sr. No. | Name | Designation | Role in a Team | Qualification | Experience |
| 1 | |||||
| 2 |
11.4 HACCP Team Roles and Responsibilities
| No | Designation | HACCP Role | HACCP Responsibility |
| 1 | Managing Partner | Member | Periodic review of HACCP, Periodic Q.C review. Customer interaction/recall recruitment. Periodic audit & review of GMP, SSOP & SOP liaisons with Govt. Authorities for inspection, approval, etc. In his absence, Director will responsible. |
| 2 | Director | Member | Periodic review of HACCP, Periodic Q.C review. Customer interaction/recall recruitment Periodic audit & review of GMP, SSOP & SOP liaisons with Govt. Authorities for inspection, approval, etc. In his absence, the Managing Director will responsible. |
| 3 | Production Manager | Member | Implementation of GMP, SSOP, and SOPS Audit review GMP, SSOP & personnel hygiene maintenance. Skill training to achieve objectives production speed. Implementation of SOP for raw material receiving /preprocessing activities & co-coordinating with purchasing center. In his absence, the Quality control in charge will be responsible. |
| 4 | Quality Assurance Incharge | Member | HACCP documentation & updating monthly audit. Reviews & training monitoring of HACCP, SSOP, SOP, QMS activities. Supervises the online inspection & in-house testing of the product. Plant approval status with EIA/MPEDA etc. Carried out the internal audit. Buyer specification for products. In his absence, Q.C technologist will be responsible. |
| 5 | Quality Assurance Executive | Member | HACCP documentation & updating monthly audit. Reviews & training monitoring of HACCP, SSOP, SOP, QMS activities. Supervises the online inspection of the product. Plant approval status with EIA/MPEDA etc. Carried out the internal audit. Buyer specification for products. In his absence, Q.C In-charge will be responsible. |
| 6 | HR Manager | Member | Recruit & training employees. Lessoning with govt. Office. Involved in factory administration. |
| 7 | Planning Manager | Member | Responsible for purchasing departmental requirement items or goods as well as stock enter of all incoming materials Responsible for quality of products been purchased. Responsible for outgoing and incoming vehicles for raw materials. Coordinate with Director, Production Manager, Q.C manager. In his absence assistant, a general store in charge will be responsible |
| 8 | Product Manager | Team Leader | Overall Supervision |
11.5 Hazard Analysis
I.Introduction
The HACCP Team conducted a brainstorming session to identify all the hazards likely to happen in each processing step. Finally, they enlisted potential hazards by considering the hazards from different angles.
- What is the likelihood of its happening?
- How severe the consequences are?
- If it happens, how many people will be affected
Scoring has given as Low=1, Medium=2 & High=3
Based on the score of each category will be multiplied to arrive at a total score of each step and categorize each step based upon the score into potential hazards or not.
- Potential hazards when score 18 or above.
- Non-potential hazards when score below 18
II.Hazard Analysis for processing steps
| Process step | Hazards | Likelihood | Severity | People affected |
| Raw material Receipt and Storage | If the raw material is stored above temp.25°C/45 % RH there is the possibility of microbial growth. The supplied raw material may contain fungal or microbial growth/pesticides. | 3 | 3 | 3 |
| Dispensing | If all processing equipment and machines are not cleaned properly, there is potential for microbial growth | 2 | 3 | 3 |
| Powder Mixing | If the environmental conditions are not controlled or personnel hygiene is not maintained, there is the probability of powder deterioration and microbial growth. | 2 | 3 | 3 |
| Capsule Filling | The correct quantity of powder should be filled in each capsule and environmental conditions if not controlled, can lead to sticky powder and conditions for microbial growth | 2 | 3 | 3 |
| Labeling and Packaging | Temperature and humidity control is essential as the exposed product could deteriorate before, best before date | 1 | 1 | 1 |
Scoring
Raw material Receipt & Storage:
Dispensing:
Powder Mixing:
Capsule Filling:
Labeling and Packaging:
By risk assessment, it is concluded that the following hazards are enlisted as potential hazards
- Raw material receipt and Storage
- Dispensing
- Powder Mixing
- Capsule Filling
11.6 Hazard Analysis
I.Introduction
The HACCP Team conducted a brainstorming session to identify all the hazards likely to happen in each processing step. Finally, they enlisted potential hazards by considering the hazards from different angles.
- What is the likelihood of its happening?
- How severe the consequences are?
- If it happens, how many people will be affected
Scoring has given as Low=1, Medium=2 & High=3
Based on the score of each category will be multiplied to arrive at a total score of each step and categorize each step based upon the score into potential hazards or not.
- Potential hazards when scoring 18 or above.
- Non-potential hazards when scoring below 18
11.7 Hazard Analysis for processing steps
| Process step | Hazards | Likelihood | Severity | People affected |
| Raw material Receipt and Storage | If the raw material is stored above temp.25°C/45 % RH there is the possibility of microbial growth. The supplied raw material may contain fungal or microbial growth/pesticides. | 3 | 3 | 3 |
| Dispensing | If all processing equipment and machines are not cleaned properly, there is potential for microbial growth | 2 | 3 | 3 |
| Powder Mixing | If the environmental conditions are not controlled or personnel hygiene is not maintained, there is the probability of powder deterioration and microbial growth. | 2 | 3 | 3 |
| Capsule Filling | The correct quantity of powder should be filled in each capsule and environmental conditions if not controlled, can lead to sticky powder and conditions for microbial growth | 2 | 3 | 3 |
| Labeling and Packaging | Temperature and humidity control is essential as the exposed product could deteriorate before, best before date | 1 | 1 | 1 |
Scoring
Raw material Receipt & Storage: 27
Dispensing: 18
Powder Mixing: 18
Capsule Filling: 18
Labeling and Packaging: 1
By risk assessment, it is concluded that the following hazards are enlisted as potential hazards
- Raw material receipt and Storage
- Dispensing
- Powder Mixing
- Capsule Filling
11.8 Hazard Analysis worksheet for RM
| Entity | Identify potential hazards introduced Controlled or Enhanced at this step | Whether it is significant or not | Justification for decision in column no .3 | What preventive measure(s) can be applied to prevent the significant hazard? | Is this step a critical control point? |
| Raw Material Receipt and Storage | Biological Presence of fungal or bacterial contamination |
Yes | The incoming quality of RM may already be contaminated even though the RM received is in sealed containers. The chances of contamination are very high if the container and the bag inside are punctured or evidence of tampering is present.
Container contains a raw material that is not contaminated and properly Sealed. Airtight polybag is inside the container |
The plastic drum with RM needs to be inspected visually for cracks. Further, the inside polybag should be received in proper condition. CoA of the manufacturer needs to be validated by independent testing |
Yes |
| Chemical | No | Container contains raw material is not contaminated if properly Sealed and comes with airtight polybag inside the container |
——– |
No | |
| Physical
Extraneous matter |
No | As long as the physical container is intact, any extraneous matter is removed by hand and then de-dusted with a vacuum cleaner before opening |
——– |
No |
11.9 Hazard Analysis Work Sheet for Packing Material
| Entity | Identify potential hazards introduced controlled or enhanced at this step | Whether it is significant or not | Justification for decision in column no .3 | What preventive measure(s) can be applied to prevent the significant hazard? | Is this step a critical control point? |
|
Packing material |
Biological Presence of pathogens | No | Food-grade packing material is used. No Biological hazards are there in the Packing material Packaging materials are stored in controlled conditions |
——————-
|
No |
| Chemical
Nil |
No | Food-grade packing material is used. No Chemical hazards are there in the Packing material Packaging materials are stored in controlled conditions. |
—————— |
No | |
| Physical
Extraneous matter |
No | Packaging materials are stored in the packing material room, properly covered with a polythene sheet. Storing & racking are done brand-wise / product-wise / lot-wise. So that mixing of brand/variety are not mixed up &is maintained following SOP |
——————- |
No |
11.10 Hazard Analysis Work Sheet for Additives
| Entity | Identify potential hazards introduced controlled or enhanced at this step | Whether it is significant or not | Justification for decision in column no .3 | What preventive measure(s) can be applied to prevent the significant hazard? | Is this step a critical control point? |
| Addtive: Syloid 244 FP | Biological Presence of pathogens | No | Only USFDA or EU-approved additives
are used. A supplier test certificate is checked. The manufacturer Grace GmBH Germany provides CoA of each batch tested as per US Pharmacopoeial standards. |
——————-
|
No |
| Chemical
Nil |
No | Only USFDA or EU-approved additives are used. A supplier test certificate is checked. The manufacturer Grace GmBH Germany provides CoA of each batch tested as per US Pharmacopoeial standards. |
——————— |
No | |
| Physical
Extraneous matter |
No | Only USFDA or EU-approved additives are used. A supplier test certificate is checked. The manufacturer Grace GmBH Germany provides CoA of each batch tested as per US Pharmacopeial standards.
Only sealed bags from the original manufacturer are used |
——————- |
No |
12. Product Profile
The manual covers details of the following products that are being processed in this facility have been covered under this manual,
| Name | Generic Description |
| Caps | HPMC Vegetarian Capsule containing Herbal Blend
Other Gelatin Shell Capsules |
| Sachet | Powdered Fruit Juice mix packed in Sachet |
13. Capsules
13.1 Product Description: Herbal Capsules
| Source of Raw Material | Capsule Shells from approved Vendors:
Raw Material Herbal Powder ingredients from approved vendors |
| Product | Herbal Capsules filled in HDPE bottles |
| Formulation | Moringa Oleifera (Leaf Powder) 100mg, Oregano Leaf Extract Powder 30mg, Rosemary Leaf Extract Powder 30mg, Pomegranate Fruit Peel Extract Powder 30mg, Shilajit Extract 40mg, Spirulina Whole Plant Powder 40mg, Amla Fruit Extract Powder 40 Mg, Turmeric Root Extract Powder 10mg, Fenugreek Seed Extract Powder 60mg, Black Pepper Fruit Extract Powder 2mg |
| Varieties | HPMC Vegetarian Capsules |
| Packing | 120 capsules filled in the HDPE Bottles with Induction Sealing and then 10 bottles covered with transparent shrink wrapping. |
| Storage conditions | 25°C and 45% RH |
| Shelf life of the product | 18 months from the production date or as per the specification of ingredients |
| Additives used | None |
| Distribution | All over India by road transportation. Packaging includes shrinking wrapping the shipper carton to prevent exposure to dust and moisture |
| Intended use by customer | The product is consumed by swallowing the capsule whole with water or other beverage of choice |
For More Pharma Updates Visit -https://pharmaguidances.com

