Technology Transfer in pharmaceutical manufacturing (WHO)
- Introduction
- Scope
- Glossary
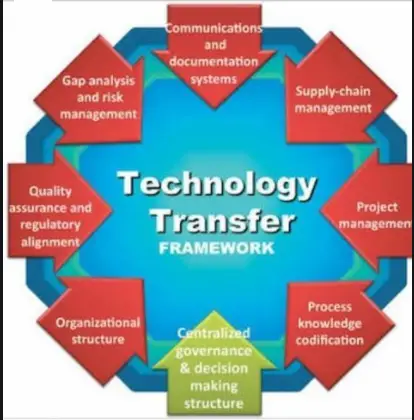
- Organization and management
- Production: transfer (processing, packaging and cleaning)
- Quality control: analytical method transfer
- Premises and equipment
- Documentation
- Qualification and validation
1.1 Transfer of processes to an alternative site occurs at some stage in the life-cycle of most products, from development, scale-up, manufacturing, production and launch, to the post-approval phase.
1.2 Transfer of technology is defined as “a logical procedure that controls the transfer of any process together with its documentation and professional expertise between development and manufacture or between manufacture sites”. It is a systematic procedure that is followed in order to pass the documented knowledge and experience gained during development and or commercialization to an appropriate, responsible and authorized party. Technology transfer embodies both the transfer of documentation and the demonstrated ability of the receiving unit (RU) to effectively perform the critical elements of the transferred technology, to the satisfaction of all parties and any applicable regulatory bodies.
1.3 Literature searches revealed little information on the subject originating from national or regional regulatory bodies.
1.4 The ever changing business strategies of pharmaceutical companies increasingly involve intra- and intercompany transfers of technology for reasons such as the need for additional capacity, relocation of operations or consolidations and mergers.
1.5 Transfer of technology requires a documented, planned approach using trained and knowledgeable personnel working within a quality system, with documentation of data covering all aspects of development, production and quality control. Usually there is a sending unit (SU), a receiving unit and the unit managing the process, which may or may not be a separate entity. For “contract manufacturing” please see good manufacturing practices (GMP) (3).
1.6 For the transfer to be successful, the following general principles and requirements should be met:
- The project plan should encompass the quality aspects of the project and be based upon the principles of quality risk management;
- The capabilities of the SU and at the RU should be similar, but not necessarily identical, and facilities and equipment should operate according to similar operating principles;
- A comprehensive technical gap analysis between the SU and RU including technical risk assessment and potential regulatory gaps, should be performed as needed;
- adequately trained staff should be available or should be trained at the RU: — regulatory requirements in the countries of the SU and the RU, and in any countries where the product is intended to be supplied, should be taken into account and interpreted consistently throughout any transfer programme project;
— there should be effective process and product knowledge transfer.
1.7 Technology transfer can be considered successful if there is documented evidence that the RU can routinely reproduce the transferred product, process or method against a predefined set of specifications as agreed with the SU.
1.8 In the event that the RU identifies particular problems with the process during the transfer, the RU should communicate them back to the SU to ensure continuing knowledge management.
1.9 Technology transfer projects, particularly those between different companies, have legal and economic implications. If such issues, which may include intellectual property rights, royalties, pricing, conflict of interest and confidentiality, are expected to impact on open communication of technical matters in any way, they should be addressed before and during planning and execution of the transfer.
1.10 Any lack of transparency may lead to ineffective transfer of technology.
1.11 Some of the principles outlined in this document may also be applicable to manufacturing investigational pharmaceutical products for clinical trials as part of research and development, but this is not the main focus of this guidance and has been excluded due to the complexity of the processes.
1.12 Some of the responsibilities outlined in this document for the SU may also be considered to be part of the management unit responsibilities.
Scope
Note: This section specifically provides for transfer of quality control (QC) methods where a technical agreement exists (SU manufacturer to RU manufacturer or SU manufacturer to RU QC laboratory).
Where no such technical agreements exist (e.g. testing by national laboratories or testing 288 for procurement agencies) a number of the points listed in section 2.4 may not be workable, and alternative approaches may be required.
2.1 This document gives guidance in principle and provides general recommendations on the activities necessary to conduct a successful intra or inter site transfer of technology as described in the Introduction to these guidelines. The intention is to address the basic considerations needed for a successful transfer in order to satisfy the regulatory authority defined for the transfer process.
2.2 The guidelines will be applied to manufacturing active pharmaceutical ingredients (APIs), manufacturing and packaging of bulk materials, manufacturing and packaging of finished pharmaceutical products (FPPs) and/or performing analytical testing.
2.3 The recommendations provided in these guidelines apply to all dosage forms but need to be adjusted on a case-by-case basis (e.g. by using risk management principles). Particularly close control of certain aspects will be required for certain formulations such as sterile products, and metered dose aerosols. WHO guidance on manufacture of specific pharmaceutical products(4,5) will be useful in this regard.
2.4 The guidelines address the following areas at the SU and the RU:
— transfer of development and production (processing, packaging and cleaning);
— transfer of analytical methods for quality assurance and quality control;
— Skills assessment and training;
— Organization and management of the transfer;
— Assessment of premises and equipment;
— Documentation; and
— Qualification and validation
. 2.5 Because each transfer project is unique, the provision of a comprehensive set of guidelines is beyond the scope of this document.
2.6 These guidelines do not provide guidance on any legal, financial or commercial considerations associated with technology transfer projects.
Organization and management
4.1 Transfer comprises an SU and an RU. In some circumstances there may be an additional unit which will be responsible for directing, managing and approving the transfer.
4.2 There is a formal agreement between the parties, which specifies the responsibilities before, during and after transfer.
4.3 Organization and management of a successful technology transfer need to ensure that the main steps have been executed and documented as described in section 1.6.
4.4 There should be a project management plan which identifies and controls all the necessary activities identified at the start of the undertaking.
4.5 The transfer protocol should list the intended sequential stages of the transfer.
The protocol should include:
— Objective;
— scope;
— key personnel and their responsibilities;
— A parallel comparison of materials, methods and equipment;
— The transfer stages with documented evidence that each critical stage has been satisfactorily accomplished before the next commences;
— Identification of critical control points;
— Experimental design and acceptance criteria for analytical methods;
— Information on trial production batches, qualification batches and process validation;
— Change control for any process deviations encountered;
— Assessment of end-product;
— Arrangements for keeping retention samples of active ingredients, intermediates and finished products, and information on reference substances where applicable; and
— Conclusion, including signed-off approval by project manager.
4.6 The SU should provide the necessary validation documentation for the process and its support functions. Usually, an established process is transferred, and such documentation is already available.
4.7 The SU should provide criteria and information on hazards and critical steps associated with the product, process or method to be transferred, to serve as a basis for a quality risk management (QRM) exercise at the RU (7–10).
4.8 The SU or third party should assess the suitability and degree of preparedness of the RU before transfer, with regard to premises, equipment and support services (e.g. purchasing and inventory control mechanisms, quality control (QC) procedures, documentation, computer validation, site validation, equipment qualification, water for pharmaceutical production and waste management).
4.9 The SU and the RU should jointly verify that the following, satisfactorily completed, validation protocols are available:
- Installation qualification (IQ) and operational qualification (OQ) data for manufacturing and packaging equipment at the RU site and analytical equipment; and
- Qualification of the rooms for both manufacture and packaging at the RU site.
4.10 The SU and the RU should jointly implement any training programmes that may be required specific to the product, process or method to be transferred, e.g. on analytical methods or equipment usage, and assess training outcomes.
4.11 The SU and the RU should jointly execute the transfer protocol according to a checklist and or flow diagram showing the sequence of steps to be carried out to effect an efficient transfer.
4.12 Any changes and adaptations made during the course of the technology transfer should be fully documented.
4.13 The SU and the RU should jointly document the execution of the transfer protocol in a transfer of technology summary in a report.
Project team
4.14 Any transfer project will be managed by a team comprising members with clearly defined key responsibilities. The team should be drawn from members of relevant disciplines from both the SU and RU sites.
4.15 The team members should have the necessary qualifications and experience to manage their particular aspect of the transfer.
Production: transfer (processing, packaging and cleaning)
5.1 The RU should be able to accommodate the intended production capacity. If possible, it should be established at the outset whether the intention is to perform single-batch manufacture, continuous production or campaigns.
5.2 Consideration should be given to the level and depth of detail to be transferred to support production and any further process development and optimization at the RU as intended under the transfer project plan.
5.3 Consideration should be given to the technical expertise, site technology and site capabilities for the RU. It should be identified upfront by the SU of any process robustness issues so that plans may be put in place at the RU.
5.4 The SU and the RU should jointly develop a protocol for the transfer of relevant information related to the process under consideration from the SU to the RU, as well as the development of a comparable process at the RU.
Starting materials
5.5 The specifications and relevant functional characteristics of the starting materials (APIs and excipients) (11,12) to be used at the RU should be consistent with materials used at the SU.
Any properties which are likely to influence the process or product should be identified and characterized.
Active pharmaceutical ingredients (API)
5.6 The SU should provide the RU with the open (applicant’s) part of the API master file (APIMF or drug master file (DMF) or active substance master file (ASMF)), or equivalent information and any relevant additional information on the API of importance for the manufacture of the pharmaceutical product.
The following are examples of the information which may typically be provided; however the information needed in each specific case should be assessed using the principles of QRM:
- Manufacturer and associated supply chain;
- Step of the API to be transferred;
- flow chart of synthesis pathway, outlining the process, including entry points for raw materials, critical steps, process controls and intermediates;
- Where relevant, definitive physical form of the API (including photomicrographs and other relevant data) and any polymorphic and solvate forms;
- Solubility profile;
- If relevant, pH in solution;
- Partition coefficient, including the method of determination;
- Intrinsic dissolution rate, including the method of determination;
- particle size and distribution, including the method of determination;
- Bulk physical properties, including data on bulk and tap density, surface area and porosity as appropriate;
- Water content and determination of hygroscopicity, including water activity data and special handling requirements;
- Microbiological considerations (including sterility, bacterial endotoxins and bioburden levels where the API supports microbiological growth) in accordance with national, regional or international pharmacopoeial requirements;
- Specifications and justification for release and end-of-life limits;
- Summary of stability studies conducted in conformity with current guidelines, including conclusions and recommendations on retest date;
- List of potential and observed synthetic impurities, with data to support proposed specifications and typically observed levels;
- Information on degradants, with a list of potential and observed degradation products and data to support proposed specifications and typically observed levels;
- potency factor, indicating observed purity and justification for any recommended adjustment to the input quantity of API for product manufacturing, providing example calculations; and
- Special considerations with implications for storage and or handling, including but not limited to safety and environmental factors (e.g. as specified in material safety data sheets) and sensitivity to heat, light or moisture.
Excipients
5.7 The Excipients (11) to be used have a potential impact on the final product.
Their specifications and relevant functional characteristics should, therefore, be made available by the SU for transfer to the RU site.
The following are examples of the information which may typically be provided; however, the information needed in each specific case should be assessed using the principles of QRM:
- Manufacturer and associated supply chain;
- Description of functionality, with justification for inclusion of any antioxidant, preservative or any excipient;
- Definitive form (particularly for solid and inhaled dosage forms);
- Solubility profile (particularly for inhaled and transdermal dosage forms);
- partition coefficient, including the method of determination (for transdermal dosage forms);
- Intrinsic dissolution rate, including the method of determination (for transdermal dosage forms);
- Particle size and distribution, including the method of determination (for solid, inhaled and transdermal dosage forms);
- Bulk physical properties, including data on bulk and tap density, surface area and porosity as appropriate (for solid and inhaled dosage forms);
- Compaction properties (for solid dosage forms);
- Melting point range (for semi-solid or topical dosage forms);
- pH range (for parenteral, semi-solid or topical, liquid and transdermal dosage forms);
- Ionic strength (for parenteral dosage forms);
- Specific density or gravity (for parenteral, semi-solid or topical, liquid and transdermal dosage forms); • viscosity and or viscoelasticity (for parenteral, semi-solid or topical, liquid and transdermal dosage forms);
- Osmolarity (for parenteral dosage forms);
- Water content and determination of hygroscopicity, including water activity data and special handling requirements (for solid and inhaled dosage forms);
- Moisture content range (for parenteral, semisolid or topical, liquid and transdermal dosage forms);
- Microbiological considerations (including sterility, bacterial endotoxins and bioburden levels where the excipient supports microbiological growth) in accordance with national, regional or international pharmacopoeial requirements, as applicable (for general and specific monographs);
- Specifications and justification for release and end-of-life limits;
- Information on adhesives supporting compliance with peel, sheer and adhesion design criteria (for transdermal dosage forms);
- Special considerations with implications for storage and or handling, including but not limited to safety and environmental factors (e.g. as specified in material safety data sheets (MSDS)) and sensitivity to heat, light or moisture; and
- Regulatory considerations, e.g. documentation to support compliance with transmissible animal spongiform encephalopathy certification requirements (where applicable).
Information on process and finished pharmaceutical products information
5.8 The SU should provide a detailed characterization of the product, including its qualitative and quantitative composition, physical description, method of manufacture, in-process controls, control method and specifications, packaging components and configurations, and any safety and handling considerations.
5.9 The SU should provide any information on the history of process development which may be required to enable the RU to perform any further development and or process optimization after successful transfer.
Such information may include the following:
- Information on clinical development, e.g. information on the rationale for the synthesis, route and form selection, technology selection, equipment, clinical tests, and product composition;
- information on scale-up activities: process optimization, statistical optimization of critical process parameters, critical quality attributes, pilot report and or information on pilot-scale development activities indicating the number and disposition of batches manufactured;
- information or report on full-scale development activities, indicating the number and disposition of batches manufactured, and deviation and change control (sometimes referred to as change management) reports which led to the current manufacturing process;
- the change history and reasons, e.g. a change control log, indicating any changes to the process or primary packaging or analytical methods as a part of process optimization or improvement; and
- information on investigations of problems and the outcomes of the investigations.
5.10 The SU should provide to the RU information on any health, safety and environmental issues associated with the manufacturing processes to be transferred, and the implications, e.g. need for gowning or protective clothing.
5.11 The SU should provide to the RU information on current processing and testing, including but not limited to:
- a detailed description of facility requirements and equipment;
- information on starting materials, applicable MSDS and storage requirements for raw materials and fi nished products;
- Description of manufacturing steps (narrative and process maps or flow charts, and or master batch records), including qualification of in processing hold times and conditions, order and method of raw material addition and bulk transfers between processing steps;
- Description of analytical methods;
- identification and justification of control strategy (e.g. identification of critical performance aspects for specific dosage forms, identification of process control points, product quality attributes and qualification of critical processing parameter ranges, statistical process control (SPC) charts);
- design space, in cases where this has been defined;
- Validation information, e.g. validation plans and reports;
- Annual product quality reviews;
- Stability information;
- An authorized set of protocols and work instructions for manufacturing; and
- Environmental conditions or any special requirement needed for the facility or equipment depending on the nature of the product to be transferred.
5.12 During the transfer process, the RU should identify any differences in facilities, systems and capabilities and communicate with the SU about these differences to understand the potential impact on ability to run the process to deliver good product quality.
Differences should be understood and satisfactorily addressed to assure equivalent product quality. Based on the information received from the SU, the RU should consider its own capability to manufacture and pack the product to the required standards and should develop relevant plant operating procedures and documentation before the start of production.
Process development at the RU should address the following tasks:
- Comparison and assessment of suitability and qualification of facility and equipment;
- Description of manufacturing process and flow of personnel and of materials at the RU (narrative and or process maps or flow charts);
- Determination of critical steps in manufacture, including hold times, endpoints, sampling points and sampling techniques;
- writing and approval of SOPs for all production operations (e.g. dispensing, granulation or blending or solution preparation, tablet compression, tablet coating, encapsulation, liquid filling, primary and secondary packaging and in-process quality control), packaging, cleaning, testing and storage;
- Evaluation of stability information, with generation of site-specific stability data if required; and
- Compliance with regulatory requirements for any changes made, e.g. in terms of batch size.
Packaging
5.13 The transfer of packaging operations should follow the same procedural patterns as those of the production transfer.
5.14 Information on packaging to be transferred from the SU to the RU includes specifications for a suitable container or closure system, as well as any relevant additional information on design, packing, processing or labelling requirements and tamper-evident and anti-counterfeiting measures needed for qualifi cation of packaging components at the RU.
5.15 For QC testing of packaging components, specifications should be provided for drawings, artwork and material (for example, glass, card or fibre board).
5.16 Based on the information provided, the RU should perform a suitability study for initial qualification of the packaging components. Packaging is considered suitable if it provides adequate protection (preventing degradation of the medicine due to environmental influences), safety (absence of undesirable substances released into the product), compatibility (absence of interaction possibly affecting medicine quality) and performance (functionality in terms of drug delivery).
Cleaning
5.17 During the manufacturing process, pharmaceutical products and APIs can be contaminated by other pharmaceutical products or APIs if the plant is processing different products. To minimize the risk of contamination and cross-contamination, operator exposure and environmental effects, adequate cleaning procedures are essential.
5.18 Cleaning procedures and their validation are site-specific. In order for the RU to define its cleaning strategy the SU should provide information on cleaning at the SU to minimize cross-contamination due to residues from previous manufacturing steps, operator exposure and environmental impact, including: — information on solubility of active ingredients, Excipients and vehicles;
— Minimum therapeutic doses of active ingredients;
— Therapeutic category and toxicological assessment; and
— existing cleaning procedures. Additional information should be provided, as appropriate and where available, e.g.:
— cleaning validation reports (chemical and microbiological);
— Information on cleaning agents used (efficacy, evidence that they do not interfere with analytical testing for residues of APIs, removal of residual cleaning agents); and
— Recovery studies to validate the sampling methodology.
5.19 Before the transfer, the SU should provide information on limits for product residues, and the rationale for limit selection.
5.20 Based on the information provided by the SU, cleaning procedures should be designed at the RU, taking into account relevant characteristics of the starting materials (e.g. potency, toxicity, solubility, corrosiveness and temperature sensitivity), manufacturing equipment design and configuration, cleaning agent and products residue.
Implementation of processing, packaging and cleaning systems
5.21 Trial batch (“demonstration batches”) are normally produced to confirm process capability before initiating formal validation. Where trial batches are produced, at a minimum, all critical processing parameters and finished product specifications should be assessed.
5.22 Once process capability has been established at the RU, assuring that the product, process or method at the RU meets predefined and justified specifications, process validation and cleaning validation can be carried out.
Quality control: analytical method transfer
6.1 Transfer of analytical methods should accommodate all the analytical testing required to demonstrate compliance of the product to be transferred with the registered specification.
6.2 Analytical methods used to test pharmaceutical products, starting materials, packaging components and cleaning (residue) samples, if applicable, should be implemented at the testing laboratory before testing of samples for process validation studies is performed by the RU. Process validation samples may be tested at the RU, the SU or a third laboratory.
6.3 A protocol defining the steps should be prepared for transfer of analytical methods. The analytical methods transfer protocol should include a description of the objective, scope and responsibilities of the SU and the RU; a specification of materials and methods; the experimental design and acceptance criteria; documentation (including information to be supplied with the results, and report forms to be used, if any); procedure for the handling of deviations; references; signed approval; and details of reference samples (starting materials, intermediates and finished products).
6.4 The SU’s responsibilities for the transfer of analytical methods are to:
- provide method-specific training for analysts and other quality control staff, if required;
- assist in analysis of QC testing results;
- define all methods to be transferred for testing a given product, starting material or cleaning sample;
- define experimental design, sampling methods and acceptance criteria;
- provide any validation reports for methods under transfer and demonstrate their robustness;
- provide details of the equipment used, as necessary (part of validation report, if available) and any standard reference samples;
- provide approved procedures used in testing; and
- review and approve transfer reports.
6.5 The RU’s responsibilities are to:
- review analytical methods provided by the SU, and formally agree on acceptance criteria before execution of the transfer protocol;
- ensure that the necessary equipment for QC is available and qualified at the RU site. The equipment used by the RU during the analytical transfer should meet appropriate specifications to ensure the requirements of the method or specification are met;
- ensure that adequately trained and experienced personnel are in place for analytical testing;
- provide a documentation system capable of recording receipt and testing of samples to the required specification using approved test methods, and of reporting, recording and collating data and designation of status (approved, rejected, quarantine);
- execute the transfer protocol;
- perform the appropriate level of validation to support the implementation of the methods; and
- generate and obtain approval of transfer reports.
6.6 Appropriate training should be provided and all training activities and outcomes should be documented.
6.7 Reference to compendial monographs (e.g. The International Pharmacopoeia , European Pharmacopoeia, British Pharmacopoeia and United States Pharmacopeia), where available, is expected.
6.8 Possible experimental designs and acceptance criteria for the main analytical testing methods are shown in Table 1.
| Possible experimental designs and acceptance criteria for analytical testing | |||||
| Test | Considerations for transfer |
Replication of tests |
Set-up | Acceptance criteria | |
| Direct | Statistically derived |
||||
| Identity | Transfer should focus on sample preparation, instruments, data interpretation. Acceptable to include in assay transfer where relevant |
One determination usually sufficient to demonstrate equivalence |
– | – | – |
| Assay for potency |
– Non-specific assay should not be used for stability testing. – Bracketing may be appropriate for multiple strengths |
At each site: 2 analysts × 3 lots, in triplicate (= 18 per site) |
Different sets of instruments and columns Independent solution preparation |
Comparison of mean and variability |
Two one sided tests with inter site differences ≤ 2% , 95% confidence |
| Content uniformity |
If method is equivalent to assay method, separate transfer is not usually required |
At each site: 2 analysts, × 1 lot (= 2 per site) |
Different sets of instruments and columns Independent solution preparation |
Mean at RU within ± 3% of mean at SU; comparison of relative st. dev |
Two one sided t-tests with inter site differences ≤ 3% , 95% confidence |
| Dissolution | Bracketing may be appropriate for multiple strengths |
6 units (12 if not routine at RU, and for extended release products) |
Mean at RU within ± 5% of mean at SU |
Compare profile (e.g. F2 ), or Compare data at Q time points as for assay |
|
| Cleaning verification (recovery of residues from surfaces) |
Confirm that same swabbing material is used at sending unit (SU) and receiving unit (RU) |
– | Use spiked samples, with levels within 3× validated st. dev. or within ± 10% of specification (whichever is the greater) |
– All samples spiked above specification should fail – 90% of samples spiked below specification should pass |
– |
| Microbiological testing (qualitative and quantitative limit tests) |
– Execute common on-site validation protocol: rationale; method identity; validation parameters; data summary; acceptance criteria; methods of compiling and analysing data; handling of out-of-specification results; follow-up requirements – Use same materials, techniques, inoculum preparation |
Validation in triplicate |
Use different lots for each validation exercise |
– Qualitative: Demonstrate recovery of microorganisms – Quantitative: Recovery levels within acceptance limits specified in protocol |
– |
| Impurity, degradation, residual solvents |
Confirm response factors for calculation relative to drug peak; – Confirm limit of quantitation at RU; – Compare chroma to grams – Compare accuracy and precision for spiking experiments |
At each site: 2 analysts × 3 lots, in duplicate (in triplicate if done together with assay) |
– Different days, different sets of instruments and columns – Use samples of similar age, homogeneity, packaging, storage – Use spiked samples if necessary |
(For low levels) Values at RU within ± 25% of values at SU, or Mean at RU within ± 0.05% of mean at SU (5%) |
(For moderately high levels) Two one sided t-tests, differences ≤ 10%, 95% confidence |
| st. dev., standard deviation. Note: numbers in the table are given as examples only and should not be considered as recommendations. |
|||||
Note that this table represents high-level guidance to apply the general principle that method transfers should account for the variability and sensitivity of the method and the specifications for the quality parameter. Alternative procedures and acceptance criteria may be applied based on science and the characteristics of the analytical method and the analyte.
Premises and equipment Premises
7.1 The SU should provide information to the RU on the layout, construction and finish of buildings and services (heating, ventilation and air conditioning (HVAC), temperature, relative humidity, water, power, and compressed air), which have an impact on the product, process or method to be transferred.
7.2 The SU should provide information on relevant health, safety and environmental issues, including:
- Inherent risks of the manufacturing processes (e.g. reactive chemical hazards, exposure limits, fi re and explosion risks);
- Health and safety requirements to minimize operator exposure (e.g. atmospheric containment of pharmaceutical dust);
- Emergency planning considerations (e.g. in case of gas or dust release, spillage, fire and firewater run-off); and
- Identification of waste streams and provisions for re-use, recycling and/ or disposal.
Equipment
7.3 The SU should provide a list of equipment, makes and models involved in the manufacture, filling, packing and or control of the product, process or method to be transferred, together with existing qualification and validation documentation.
Relevant documentation may include:
— Drawings;
— Manuals;
— Maintenance logs;
— Calibration logs; and
— Procedures (e.g. regarding equipment set-up, operation, cleaning, maintenance, calibration and storage).
7.4 The RU should review the information provided by the SU together with its own inventory list including the qualification status (IQ, OQ, PQ) of all equipment and systems, and perform a side-by-side comparison of equipment at the two sites in terms of their functionality, makes, models and qualification status.
7.5 The RU should perform a gap analysis to identify requirements for adaptation of existing equipment, or acquisition of new equipment, or a change in the process, to enable the RU to reproduce the process being transferred.
GMP requirements should be satisfied and intended production volumes and batch sizes (e.g. same, scaled-up or campaign) should be considered.
Factors to be compared include:
— Minimum and maximum capacity;
— Material of construction;
— Critical operating parameters;
— Critical equipment components (e.g. filters, screens, and temperature/ pressure sensors);
— Critical quality attribute; and
— range of intended use.
7.6 The facility- and building-specific location of all equipment at the RU should be considered at the time of drawing up process maps or flow charts of the manufacturing process to be transferred, including flows of personnel and material.
7.7 The impact of manufacturing new products on products currently manufactured with the same equipment should be determined.
7.8 Any modification of existing equipment that needs to be adapted to become capable of reproducing the process being transferred should be documented in the transfer project plan.
Documentation
8.1 The documentation required for the transfer project itself is wide ranging. Examples of documentation commonly required are summarized in Table 2.
| Examples of documentation for transfer of technology (TOT) | ||
| Key task | Documentation provided by SU | Transfer documentation |
| Project definition | Project plan and quality plan (where separate documents), protocol, risk assessments, gap analysis |
Project implementation plan TOT protocol |
| Quality agreement
Facility assessment |
Plans and layout of facility, buildings (construction, finish) Qualification status (DQ, IQ, OQ) and reports |
Side-by-side comparison with RU facility and buildings; gap analysis Qualification protocol and report |
| Health & Safety assessment |
Product-specific waste management plans Contingency plans |
– |
| Skill set analysis and training |
SOPs and training documentation (product-specific operations, analysis, testing) |
Training protocols, assessment results |
| Analytical method transfer |
Analytical method specifications and validation, including in-process quality control |
Analytical methods transfer protocol and report |
| Starting material evaluation |
Specifications and additional information on APIs, excipients |
– |
| Equipment selection and transfer |
Inventory list of all equipment and systems, including makes, models, qualification status (IQ, OQ, PQ) Drawings, manuals, logs, SOPs (e.g. set-up, operation, cleaning, maintenance, calibration, storage) |
Side-by-side comparison with RU equipment (makes, models, qualification status) Gap analysis Qualification and validation protocol and report |
| Process transfer: manufacturing and packaging |
Reference batches (clinical, dossier, bio batches) Development report (manufacturing process rationale) History of critical analytical data Rationale for specifications Change control documentation Critical manufacturing process parameters Process validation reports Drug master fi le API validation status and report(s) Product stability data Current master batch manufacturing and packaging records List of all batches produced Deviation reports Investigations, complaints, recalls Annual product review |
History of process development at RU Experiences at RU should be recorded for future reference Provisional batch manufacturing document (RU to develop) Provisional batch packaging document (RU to develop) Description of process at RU (narrative, process map, flow chart) Process validation protocol and report |
| Cleaning | Cleaning validation, including: Solubility information; therapeutic doses; category (toxicology); existing cleaning SOPs; validation reports — chemical and micro; agents used; recovery study |
Product- and site-specific cleaning SOPs at RU Cleaning validation protocol and report |
| DQ, design qualification; IQ, installation qualification; OQ, operational qualification; API, active pharmaceutical ingredient; SOPs, standard operating procedures; RU, receiving unit. | ||
8.2 The documented evidence that the transfer of technology has been considered successful should be formalized and stated in a technology transfer summary report. That report should summarize the scope of the transfer, the critical parameters as obtained in the SU and RU (preferably in a tabulated format) and the final conclusions of the transfer. Possible discrepancies should be listed and appropriate actions, where needed, taken to resolve them.
Qualification and validation General
9.1 The extent of qualification and or validation to be performed should be determined on the basis of risk management principles.
9.2 Qualification and validation should be documented.
Reference: WHO guidelines on transfer of technology in pharmaceutical manufacturing (Annex 7)