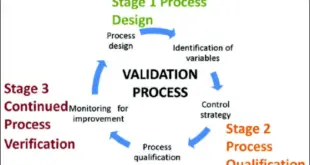
ICH Q8/Q9/Q10 1.1 For General Clarification Date of Approval Questions Answers 1.0 June 2009 Is the minimal approach accepted by regulators? Yes. The minimal approach as defined in Q8(R2) (sometimes also called ‘baseline’ or ‘traditional’ approach) is the expectation that is to be achieved for a fully acceptable submission. However, …
Read More »Guidance Document Cleaning Validation
Guidance Document Cleaning Validation Scope Introduction Principles Validation of Cleaning Processes Equipment and Personnel Microbiological Considerations Documentation Analytical Methods Sampling, Rinsing, Rinse Samples and Detergents Establishment of Limits Change Control/revalidation References 3.1 The objective of the cleaning validation is to verify the effectiveness of the cleaning procedure for removal of …
Read More »Technology Transfer in pharmaceutical manufacturing (WHO)
Technology Transfer in pharmaceutical manufacturing (WHO) Introduction Scope Glossary Organization and management Production: transfer (processing, packaging and cleaning) Quality control: analytical method transfer Premises and equipment Documentation Qualification and validation 1.1 Transfer of processes to an alternative site occurs at some stage in the life-cycle of most products, from development, …
Read More »Process Validation Critical Parameters
Process Validation Critical Parameters Process Validation(FDA Definition) Establishing Documented Evidence, Which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.” Steps in Validating a Process: Develop validation protocol Conduct installation qualification Conduct operational qualification Conduct performance qualification …
Read More »Equipment Status log
Equipment Status log OBJECTIVE: To lay down a procedure of the maintenance of equipment sequential log. SCOPE: This SOP is applicable to all equipment and machines of all manufacturing departments RESPONSIBILITY: Production and Engineering officer/above shall be responsible to follow the procedure mentioned in this SOP. ACCOUNTABILITY : Concerned Department Head and QA Head …
Read More »Cleaning of general item
Cleaning of general item OBJECTIVE: To lay down a procedure to describe the steps to be followed for cleaning general items. SCOPE: Applicable for cleaning all HDPE / SS containers, utensils, scoops, sampling tools and different cleaning aids RESPONSIBILITY: Housekeeping workers, Production Supervisors, Operators, IPQA personnel shall be responsible to follow the …
Read More »NON-CONFORMANCE RESULTS
NON-CONFORMANCE RESULTS OBJECTIVE: To lay down the procedure for define the necessary action against the non-conformance of a product. SCOPE: This procedure is applicable for information, investigation and control for any kind of non- conformance noticed or confirmed in Production, Stores on in others area for its proper investigation RESPONSIBILITY: Officer – …
Read More »Prevention and Product Mix Up
Prevention and Product Mix Up OBJECTIVE: To lay down the Procedure for prevention of Product mix-up. SCOPE: This procedure is applicable for the control and prevention of product mix up during and after the manufacturing of the product . RESPONSIBILITY: QA officer and Production Officer shall be responsible for the …
Read More »GUIDE TO INSPECTIONS VALIDATION OF CLEANING PROCESSES
INTRODUCTION To establish inspection consistency and uniformity by discussing practices that has been found acceptable (or unacceptable). The cleaning validation – to validate the process and collect the scientific data that prove the system consistently does as expected and produce a result that consistently meets predetermined specifications. This guide is …
Read More »Non-sterile process validation
Non-sterile process validation Process validation data should be generated for all products to demonstrate the adequacy of the manufacturing process at each site of manufacture. The validation should be carried out in accordance with good manufacturing practices (GMP) and data should be held at the manufacturing location and made available …
Read More »URS of De-Dusting Machine
URS of De-Dusting Machine S. No. Table of Contents 1.0 General 2.0 Salient Features 3.0 Operational Requirements 4.0 Utilities 5.0 Maintenance 6.0 Commissioning and Documentation 7.0 Training 8.0 Packaging 9.0 Deviations 10.0 Delivery TECHNICAL S. No. Parameters Required Specifications 1. 1.1 General Equipment No. 1.2 Description Portable unit for Tablets …
Read More »SOP on house keeping, cleaning and sanitization of general area in Pharmaceutical company
SOP on housekeeping, cleaning, and sanitization of the general area in a Pharmaceutical company. Objective: To lay down a procedure for Good House Keeping, cleaning, and sanitization of the General Area. Scope: This procedure is applicable for cleaning and sanitization of General areas other than the manufacturing area of the …
Read More »QUALITY SYSTEMS MODEL (Resources)IN PHARMACEUTICAL INDUSTRY (Part – II)
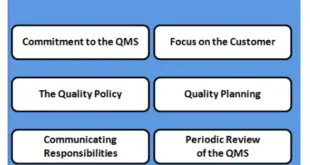
QUALITY SYSTEMS MODEL The goal is to describe a model for use in pharmaceutical manufacturing that can help manufacturers comply with the CGMP regulations. It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources. However, we believe …
Read More »Pharma WhatsApp Groups for Latest Pharma updates
Dear All Please find the Pharma WhatsApp Groups for Latest Pharma Jobs, Pharma news, and Pharma Posts. Please Click the below link and Join the group easily Pharma Post and Jobs 01 https://chat.whatsapp.com/Dy1GkgHZloYJPsfzIC6tnZ Pharma posts and jobs 25 https://chat.whatsapp.com/JZD6QCCtaGpGdWYu3oU3iy
Read More »QUALITY SYSTEMS MODEL IN PHARMACEUTICAL INDUSTRY (Part – I)
QUALITY SYSTEMS MODEL The goal is to describe a model for use in pharmaceutical manufacturing that can help manufacturers comply with the CGMP regulations. It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources. However, we believe …
Read More »CGMPS AND THE CONCEPTS OF MODERN QUALITY SYSTEMS
CGMPS AND THE CONCEPTS OF MODERN QUALITY SYSTEMS Quality should be built into the product, and testing alone cannot be relied on to ensure product quality. Several key concepts are critical for any discussion of modern quality systems. The following concepts are used throughout this guidance as they relate to …
Read More »