Reference-Standard and Working Standards The US Food and Drug Administration defines a reference-standard material as a “highly purified compound that is well characterized” (1). The US Pharmacopeia (USP) defines reference-standard materials as “highly characterized specimens of drug substances, excipients, reportable impurities, degradation products, compendial reagents, and performance calibrators” (2). Scientists …
Read More »REMEDIATION OF DATA INTEGRITY FAILURES
REMEDIATION OF DATA INTEGRITY FAILURES Data Integrity issues responding – Consideration should be primarily given to resolving the immediate issues identified and assessing the risks associated with the data integrity issues. The response by the company in question should outline the actions taken as part of a remediation plan. Responses …
Read More »Classification of data integrity deficiencies
Classification of data integrity deficiencies Data integrity to aid consistency in reporting and classification of data integrity deficiencies. Deficiencies relating to data integrity failure may have varying impacts on product quality. Prevalence of the failure may also vary between the actions of a single employee to an endemic failure throughout …
Read More »Bung Processor / Autoclave
Bung Processor / Autoclave Definition of Autoclave An autoclave is a pressure chamber used to carry out industrial processes requiring elevated temperature and pressure different to ambient air pressure. Autoclave is used in medical application to perform sterilization, and in the chemical industry to cure coatings, vulcanise rubber and for …
Read More »Turn Tables Boosting Efficiency : Streamlining Material Handling and Process Optimization
Turn Tables Boosting Efficiency: Streamlining Material Handling and Process Optimization Turn tables, also known as rotary tables or revolving tables, are versatile and innovative equipment that revolutionize material handling and process optimization. These rotating platforms enable smooth and controlled movement of products, components, or equipment, enhancing efficiency, reducing manual labor, …
Read More »HPLC Interview Questions
HPLC HPLC (High-Performance Liquid Chromatography) is a powerful analytical technique used for separating, identifying, and quantifying components in a mixture. It operates on the principles of liquid chromatography, with a liquid mobile phase carrying the sample through a stationary phase. The distinguishing feature of HPLC is the application of high …
Read More »NITROGEN GAS DISTRIBUTION SYSTEM
NITROGEN GAS DISTRIBUTION SYSTEM WELDING AND CLEANING DETAILS CERTIFICATION Welder will be certified to a qualified welding procedure to be followed. Welder shall be certified in the use of the specific equipment and material being used in the welding process as per agreement with client. 10% of all orbital welded …
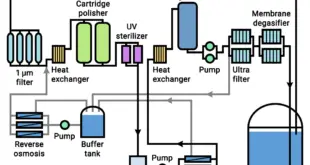
Read More »PURIFIED WATER
PURIFIED WATER WELDING AND CLEANING DETAILS CERTIFICATION Welder will be certified to a qualified welding procedure to be followed. Welder shall be certified in the use of the specific equipment and material being used in the welding process as per agreement with client. 10% of all orbital welded connections and …
Read More »QA Interview Frequently Asked Questions
MOST FREQUENTLY ASKED QUESTIONS What parameters are considered during the performance qualification of HVAC? The following parameters are to be considered during the performance qualification of HVAC: 1. Calibration test certificates of instruments 2. Training records of the validation team 3. Pressure drop across the HEPA & fine filters 4. …
Read More »Microbiological Efficacy of the Cycle
Microbiological Efficacy of the Cycle Validation studies that demonstrate the efficacy (lethality) of the production cycle need to estabilished. A sterility assurance of 10 or -6 better should be demonstrated for any terminal sterilization process. The level of sterility assurance should be demonstrated for all parts of the drug product …
Read More »Microbiological Monitoring of the Environment
Microbiological Monitoring of the Environment The microbiological monitoring program shall be performed during routine production and media fills and procedure for microbiological monitoring program are clearly defined in SOP. Microbiological monitoring – frequency of monitoring, type of monitoring, sites monitored, alert and action level specifications, and clearly defined procedure for …
Read More »TOOLS FOR QUALITY IMPROVEMENT PART – I
TOOLS FOR QUALITY IMPROVEMENT PART – I teams of people working together on projects in order to achieve pre-defined quality objectives or Eliminate the root cause(s) of a specific problem is an integral part of ST culture. To ensure that all these team members are sufficiently equipped to undertake their …
Read More »VIAL WASHING MACHINE
VIAL WASHING MACHINE An automatic rotary vial washing machine with an integral washing tank for recycled water, with multiple washing stations, is designed to clean the glass vials internally and externally with different washing media in six stages at the rated output of 120 vials per minute. Operation by the …
Read More »FDA – Warning Letter ,Dated MAY 23
FDA 483 Warning Letter Dated MAY 23, 2022 This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations for finished pharmaceuticals. See Title 21 Code of Federal Regulations (CFR), parts 210 and 211 (21 CFR parts 210 and 211). Because your methods, facilities, or controls for manufacturing, …
Read More »Sterilization and depyrogenation tunnel
Sterilization and depyrogenation tunnel
Read More »Question and Answer Part -1
Question and Answer Part -1 Question – 1: What is Media fill f Aseptic Process Simulation (APS)? Answer: A media fill or Aseptic Process Simulation (APS) is the performance of an aseptic manufacturing procedure using a sterile microbiological growth medium, in place of the drug solution, to test whether the …
Read More »FDA – Warning Letter -JUNE 14
FDA 483 Warning Letter Dated JUNE 14, 2022 1. Failure to perform repackaging of API under appropriate CGMP to avoid potential cross-contamination. During your API repackaging and relabeling operations, including highly potent drugs such as testosterone, estradiol, betamethasone, tamoxifen, and opioids, your firm failed to conduct adequate cleaning validation studies …
Read More »ASEPTIC PROCESSES VALIDATION AS PER PIC/S
ASEPTIC PROCESSES VALIDATION AS PER PIC/S Validation of aseptic processes relies upon prospective, concurrent, and retrospective validation as well as re-validation. Prospective studies include installation and operational qualification for a new or renovated facility as well as product simulation studies and a prospective process validation with the original product according …
Read More »FDA – Warning Letter – 10 JUNE
FDA – Warning Letter – 10 JUNE 2022 This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations for finished pharmaceuticals 1. The company failed to thoroughly investigate any unexplained discrepancy or failure of a batch or any of its components to meet any of its specifications, …
Read More »FDA – Warning Letter -JUNE 22
FDA 483 Warning Letter Dated JUNE 22, 2022 1. Each batch of drug product, appropriate laboratory determination of satisfactory conformance to final specifications for the drug product, including the identity and strength of each active ingredient, prior to release (21 CFR 211.165(a)) and the company failed to prove this procedure. …
Read More »