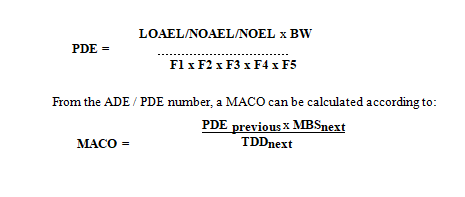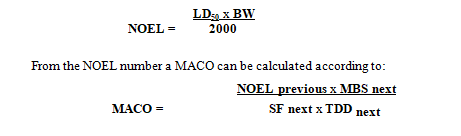MOST FREQUENTLY ASKED QUESTIONS
What parameters are considered during the performance qualification of HVAC?
The following parameters are to be considered during the performance qualification of HVAC:
1. Calibration test certificates of instruments
2. Training records of the validation team
3. Pressure drop across the HEPA & fine filters
4. Air velocity measurement & calculation of Air changes
5. Integrity test of HEPA filter
6. Differential pressure test
7. Temperature & Relative Humidity test
8. Air flow direction test
9. Cleanliness class verification (Non-viable particle count)
10. Sound level test
11. Light level test
12. Air-borne viable particle monitoring
13. Recovery Study
What are the contents in the Batch Manufacturing Records?
BPCR contains the following contents, but not limited:
1. Product Name 2. Stage
3. BMR Document Number
4. MPCR Reference Number
5. Batch Number
6. Date of Manufacturing
7. Date of Expiry/Re-test
8. Batch release details
9. List of equipment’s used
10. List of raw materials & Quantity with UOM
11. General instructions, Control & Safety instructions
12. Detailed step wise written manufacturing procedures
13. Actual results record for critical process parameters
14. Identity of In-process & Laboratory test results
15. Signatures of person performing details along with supervising details
16. Description of Packaging details
17. Yield calculation
18. Representative of labels for intermediates / raw materials
19. Deviation details
20. Batch starting & completion date
Which instrument is used for the measuring of RPM?
Techo meter is used for the measurement of RPM.
Why three batches consider for the validation?
Because of First one is for information, Second one is for confirmation and Third one is for
evidence.
If one batch is failed during the validation, then what will you do for completion of validation?
When a quality parameter fails with respect to the specification, a deviation report shall be raised and the investigation shall be conducted immediately for the identification of failure.
If the reason for failure is identified, one more consecutive batch shall be considered for the validation run by taking preventive actions to avoid those failures (If necessary revise the MPCR and BMR).
If the reason is unidentified, another three consecutive batches shall be taken for validation
What are specifications of Purified water as per any pharmacopoeia?
| Tests | Ph. Eur. |
| Description | Clear, colorless liquid |
| Acidity /Alkalinity | The solution is not colored red/The solution is not colored blue. |
| Oxidisable substances | The solution remains faintly pink |
| Chlorides | The solution shows no change in appearance for at least 15 min |
| Sulphates | The solution shows no change in appearance for at least 1 hour |
| Ammonium | Maximum 0.2 ppm. |
| Calcium and magnesium | A pure blue colour is produced. |
| Residue on evaporation | Maximum 0.001 per cent |
| Aluminum | Maximum 10 ppb |
| Nitrates | NMT 0.2 ppm |
| Heavy Metals | NMT 0.1 ppm |
| Conductivity (At 25˚C) | NMT 5.1ms.cm-1 |
| Total viable aerobic count | NMT100 CFU /ml |
| Pathogens : E. coli Salmonella Pseudomonas Staphalococcus aureus |
Absent Absent Absent Absent |
Write the different storage conditions as per any guidelines (specify the name of guideline)?
The different storage conditions are given below as per USP:
- Freezer : -25°C to -10°C
- Cold : Any temperature not exceeding 8°C
- Refrigerator : Between 2°C and 8°C
- Cool : 8°C to 15°C
- Room temp. : The temperature at prevailing working area.
- CRT : 20°C to 25°C
- Warm : 30°C to 40°C
- Excessive heat : Above 40°C
What is HACCP?
HACCP : Hazard Analysis Critical Control Point
What is OHSAS?
OHSAS : Occupational Health & Safety Assessment Series
What is room temperature?
The temperature at the prevailing working area
What is calibration?
The demonstration that a particular instrument or device produces results within specified limits by comparison with results produced by a reference or traceable standard over an appropriate range of measurements
METHODS OF CALCULATING ACCEPTANCE CRITERIA IN CLEANING VALIDATION?
- Acceptance Criteria Using Health-Based Data
- Acceptance criteria based on Therapeutic Daily Dose
- Acceptance criteria based on LD50
- General Limit as acceptance criteria (10 PPM)
How to calculate Maximum Allowable Carryover (MACO) for cleaning Validation?
- Acceptance Criteria Using Health-Based Data
The Maximum Allowable Carryover (MACO) should be based upon the Acceptable Daily Exposure (ADE) or Permitted Daily Exposure (PDE) when this data is available. The principle of MACO calculation is that you calculate your acceptable carry-over of your previous product, based upon the ADE / PDE, into your next product.
Procedure
PDE (Permitted Daily Exposure) according to the following equations and use either result for the calculation of the MACO.
MACO Maximum Allowable Carryover: Acceptable transferred amount from the previous product into your next product (mg)
PDE Permitted Daily Exposure (mg/day)
NOAEL No Observed Adverse Effect Level (mg/kg/day)
LOAEL Low Observed Adverse Effect Level (mg/kg/day)
NOEL No Observed Effect Level (mg/kg/day)
BW Is the weight of an average adult (e.g. 50 kg)
UFc Composite Uncertainty Factor: a combination of factors which reflects the inter-individual variability, interspecies differences, sub-chronic-to-chronic extrapolation, LOEL-to-NOEL extrapolation, database completeness.
MF Modifying Factor: a factor to address uncertainties not covered by the other factors
PK Pharmacokinetic Adjustments
F1-F5 Adjustment factors to account for uncertainties. Refer to EMA Guidance 2 for further explanation.
TDD next Standard Therapeutic Daily Dose for the next product (mg/day)
MBSnext Minimum batch size for the next product(s) (where MACO can end up) (mg)
Instead of calculating each potential product change situation, the worst-case scenario can be chosen. Then a case with the most active API (lowest ADE or PDE) is chosen to end up in the following API with the smallest ratio of batch size divided with TDD (MBS/TDD ratio).
If OEL data is available, the ADE or PDE can be derived from the OEL.
- Acceptance criteria based on Therapeutic Daily Dose
When limited toxicity data is available and the Therapeutic Daily Dose (TDD) is known, this calculation may be used. It is used for final product changeover API Process —A to API Process —B.
Procedure
Establish the limit for Maximum Allowable Carryover (MACO) according to the following equation.
MACO Maximum Allowance Carryover: acceptable transferred amount from the previous product into your next product (mg)
TDDprevious Standard Therapeutic Daily Dose of the investigated product (in the same dosage form as TDDnext) (mg/day)
TDD next Standard Therapeutic Daily Dose for the next product (mg/day)
MBSnext Minimum batch size for the next product(s) (where MACO can end up (mg)
SF Safety factor (normally 1000 is used in calculations based on TDD).
- Acceptance criteria based on LD50
In cases where no other data is available (e.g. ADE, PDE, OEL, TDD,…) and only LD50 data is available (e.g. chemicals, intermediates, detergents, …), the MACO can be based upon LD50 data.
Procedure
Calculate the so-called NOEL number (No Observable Effect Level) according to the following equation and use the result for the establishment of MACO.
MACO Maximum Allowance Carryover: acceptable transferred amount from the previous product into your next product (mg)
NOEL previous No Observed Effect Level (mg/day)
LD50 Lethal Dose 50 in mg/kg animal. The identification of the animal (mouse, rat etc.) and the way of entry (IV, oral etc.) is important (mg/kg)
BW Is the weight of an average adult (e.g. 50 kg) (kg)
2000 2000 is an empirical constant
TDDnext Standard Therapeutic Daily Dose for the next product (mg/day)
MBSnext Minimum batch size for the next product (s) (where MACO can end up)
SF next Safety factor
The safety factor (SF) varies depending on the route of administration (see below). Generally, a factor of 200 is employed when manufacturing APIs to be administered in oral dosage forms.
|
Safety factors:
|
Topicals | 10 – 100 |
| Oral products | 100 – 1000 | |
| Parenterals | 1000 – 10 000 |
- General Limit as acceptance criteria
If MACO calculations result in unacceptably high or irrelevant carryover figures, or toxicological data for intermediates are not known, the approach of a general limit may be suitable. Companies may choose to have such an upper limit as a policy. The general limit is often set as an upper limit for the maximum concentration (MAXCONC) of a contaminating substance in a subsequent batch.
Procedure
Establish MACO ppm, based on a general limit, using the following equations.
MACO ppm = MAXCONC x MBS
MACO ppm Maximum Allowable Carryover: acceptable transferred amount from the investigated product (“previous”). Calculated from general ppm limit.
MAXCONC General limit for maximum allowed concentration (kg/kg or ppm) of “previous” substance in the next batch.
MBS Minimum batch size for the next product(s) (where MACO can end up)
E.g. for a general limit of 100 ppm: MACO = 0.01% of the minimum batch size (MBS), and for a general limit of 10 ppm: MACO = 0.001% of the minimum batch size (MBS).
A general upper limit for the maximum concentration of a contaminating substance in a subsequent batch (MAXCONC) is often set to 5-500 ppm (100 ppm in APIs is very frequent) of the previous product into the next product depending on the nature of products produced from the individual company (e.g. toxicity, pharmacological activity etc).
The Threshold of Toxicological Concern (TTC) concept could be applied to intermediates or API’s with no clinical (e.g. early development) or toxicological data. This concept includes three categories of products with limited or no data:
- Products that are likely to be carcinogenic;
- Products that are likely to be potent or highly toxic;
- Products that are not likely to be carcinogenic, potent or highly
The corresponding ADE’s recommended for these three categories are 1, 10, 100 µg/day, respectively.
Note – If you decide to employ the concept of levels of cleaning (ref. section 5), then different safety factors (ppm limits) may be used for different levels. Especially if the product cleaned out is within the same synthetic chain and covered by the specification of the API, much higher (qualified) levels are acceptable.
What test should be performed during the validation of the Vial Washing machine?
- Estimation of washing media (Recycled water, Purified water, and WFI) consumption.
- Sodium Chloride Spiked Vials Challenge Test
- Particulate Matter Spiked Vials Challenge Test
- Endotoxin Spiked Vials Challenge Test
What test should be performed during the validation of the De-pyrogination Tunnel?
- Measurement of Airflow Velocity
- Differential Pressure Test
- Installed Filter System Integrity and Leakage Test (DOP Test)
- Airflow Pattern Test
- Non-Viable Particle Count Test
- Heat Distribution Study For Empty Chamber
- Heat Penetration Study For Load Chamber
What test should be performed during the validation of the H.P.H.V. STEAM STERILIZER?
| 1 | Steam qualification tests | ·Steam non-condensable gases test
· Steam superheat test · Steam dryness test |
| 2 | Vacuum Leak test | ·Vacuum leak test empty chamber
·Vacuum leak test Temperature sensors connected. · Vacuum leak test temperature sensors removed. |
| 3 | Empty Chamber Heat Distribution study | · With temperature mapping probes at different locations of the sterilizer chamber.
· Bio-Challenge studies shall use Bacillus stearothemophilus spore strips (containing 106 or more spores per strip) during the heat distribution studies. |
| 4 | Bowie –Dick Test | 3 Trials on 3 different days |
| 6 | Loaded Chamber heat penetration studies
(The following are the fixed standard load patterns selected for qualification of the sterilizer) |
|
| ·Aseptic area Garments
·Filling machine parts ·Filtration accessories ·Rubber stopper holding canisters ·Rubber stoppers ·Media vessels with WFI water
|
· Temperature mapping probes shall be placed inside the innermost part (assumed to be difficult to attain sterilization temperature i.e. cold spot) of the load. | |
| · Bio-Challenge studies shall use Bacillus stearothemophilus spore strips (containing 106 or more spores per strip) during the heat penetration studies. | ||
| · Estimation of the FO value achieved during the sterilization hold period at each temperature-mapping probe. | ||
How many types of cycles run in the H.P.H.V. STEAM STERILIZER during routine operation?.
-
- Vacuum leak test.
- This cycle is used to assure the sterilizer chamber integrity towards leakage.
- Bowie-Dick test.
- This cycle is used to ensure steam penetration in to the packs is appropriate.
- Standard cycle.
- Gravity displacement steam sterilization cycle.
- HPHV cycle.
- High-Pressure High Vacuum sterilization cycle with vacuum pulses.
- Vacuum leak test.
What is the operating cycle of a porous load of H.P.H.V. STEAM STERILIZER?
Operating cycle of a porous load sterilizer normally five stages
-
- Air removal – Sufficient air is removed from the chamber and the load to permit the attainment of the sterilization conditions.
- Steam admission – Steam is admitted to the chamber until the specified sterilization temperature is attained throughout the chamber and load.
- Holding time – The temperature throughout the chamber and load is maintained within the sterilization temperature band for the appropriate holding time.
- Drying – Steam is removed from the chamber and the chamber pressure is reduced to permit the evaporation of condensate from the load by prolonged evacuation.
- Air admission – Air is admitted to the chamber until the chamber pressure approaches atmospheric pressure.