SOP and Guideline for Hold-Time Studies of Tablets
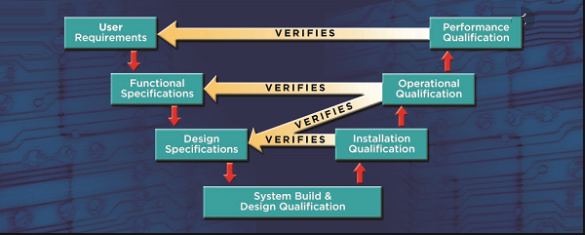
SOP and Guideline for Hold-Time Studies of Tablets These guidelines focus primarily on aspects that should be considered in the design of the hold-time studies during the manufacture of non-sterile solid dosage forms. Many of the principles described here also apply to other dosage forms such as liquids, creams, and ointments. These guidelines do not…
Read More “SOP and Guideline for Hold-Time Studies of Tablets” »