SOP FOR BUBBLE POINT TEST
Introduction
Membrane filters have been used successfully for many years to remove yeast, bacteria and particulate from fluid streams. The ultimate integrity test for a sterilizing grade membrane filter is the bacterial challenge. Unfortunately, this is a destructive test; the filter cannot be used afterwards to filter a product. As a result, one of the most important aspects of the use of filters to remove bacteria is to have a non-destructive integrity test, which is correlated to the production of effluent in a bacterial challenge test. It is this correlation, rather than the nondestructive integrity test alone, that provides assurance that the filter will perform as intended. During production of sterile product, the filter should be subjected to such an integrity test before and after filtration. This is done to ensure that the filter meets specification, is properly installed and intact during filtration, and to confirm the rating of the filter.
Ciprofloxacin, Fluocinolone and Clotrimazole Cream
There are 3 major tests used to determine the integrity of a membrane filter: the Bubble Point Test, the Forward Flow Test, and the Pressure Hold Test. All three tests are based on the same physics, the flow of a gas through a liquid-wetted membrane under applied gas pressures. They differ in which part of the flow/pressure spectrum they examine. In this paper, we will focus on the Bubble Point Test.
Background
A bubble point test is a test designed to determine the pressure at which a continuous stream of bubbles is initially seen downstream of a wetted filter under gas pressure. To perform a Bubble Point Test, gas is applied to one side of a
wetted filter, with the tubing downstream of the filter submerged in a bucket of water. The filter must be wetted uniformly such that water fills all the voids within the filter media. When gas pressure is applied to one side of the membrane, the test gas will dissolve into the water, to an extent determined by the solubility of the gas in water. Downstream of the filter, the pressure is lower. Therefore the gas in the water on the downstream side is driven out of solution. As the applied upstream gas pressure is increased, the diffusive flow downstream increases proportionally. At some point, the pressure becomes great enough to expel the water from one or more passageways establishing a path for the bulk flow of air.
As a result, a steady stream of bubbles should be seen exiting the submerged tubing. The pressure at which this steady stream is noticed is referred to as the bubble point.
Bubble Point Test Procedure
A forward bubble point integrity test is a procedure which measures the pressure needed to be applied to the upstream side of a filter causing bulk or open pore flow through the largest pores of a wetted filter. This measurement is taken from the downstream side of the filter housing where a flexible piece of tubing has
been attached and the other end submerged into a beaker of water. The bubble point is indicated by vigorous bubbling from the tubing. The accuracy of this test will rely on the operator’s ability to successful recognize this point.
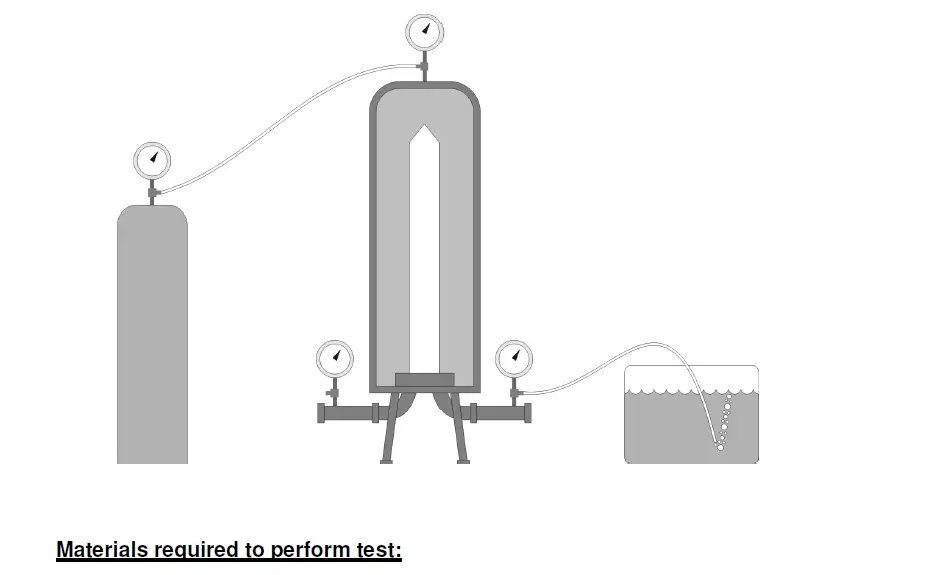
Compressed air or nitrogen
Pressure regulator
Filter, Filter housing
Hose barbs
Beaker
Tubing
Filter adapters
Test Method
1. Record the filter part number(s), lot number, and product information. Also include physical observations.
2. Wet the filter to be tested with water.
3. Place the wetted filter in the appropriate housing.
4. Connect the outlet fitting from the compressed air pressure regulator to the upstream side of the test filter. Check that the gauge which is connected to the pressure regulator has subdivisions of at least 0.5 psig, and has the
capacity to measure up to 100 psig. A digital pressure gauge can also be used.
5. Connect the outlet fitting from the compressed air pressure regulator to the upstream side of the test filter.
6. Connect a piece of flexible tubing from the downstream port of the test filter into a beaker filled with water.
7. Starting from zero pressure, gradually increase the pressure to the test filter using the pressure regulator.
8. Observe the submerged end of the tubing for the production of bubbles as the upstream pressure is slowly increased in 0.5 psig increments. Note the rate that the bubbles appear for the end of the submerged tube.
9. The bubble point of the test filter is reached when bubbles are produced from the tube at a steady rate. Record the pressure to the nearest 0.5 psig as indicated on the pressure gauge.
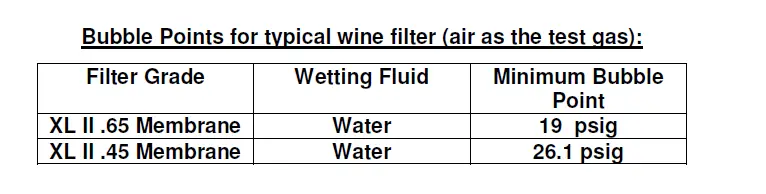
If the recorded pressure is greater than or equal to the minimum expected bubble point listed above, the filter is integral. If the recorded pressure is lower than the minimum bubble point listed above, the filter has failed the integrity test.
Test Considerations
1. Ensure that the filter is thoroughly and uniformly wet such that all the pores are filled with water. Failure to wet the filter may result in premature air flow resulting in false failure.
2. Diffusive flow of air through the filter will occur at pressures lower than the bubble point. Ensure that the pressure recorded is in fact the pressure at which a steady stream of bubbles is noted.
3. If failure occurs open the filter housing to ensure that the filter is installed correctly in the housing. Replace the housing cover and retest. If another failure is recorded remove and dispose of the filter element.

