Organization and Personnel in Pharma industry as per USFDA Responsibilities of quality control unit (§ 211.22) in Pharma industry (a) There shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and …
Read More »Production and Process Controls in Pharma industry as per USFDA
Production and Process Controls in Pharma industry as per USFDA Written procedures; deviations (21 CFR 211.100). Charge-in of components (21 CFR 211.101). Calculation of yield (21 CFR 211.103). Equipment identification (21 CFR 211.105). Sampling and testing of in-process materials and drug products (21 CFR 211.110). Time limitations on production (21 …
Read More »Definitions as per 21 CFR 210 (USFDA) in Pharma company
Definitions as per 21 CFR 210 (USFDA) in Pharma company (a) The definitions and interpretations contained in section 201 of the act shall be applicable to such terms when used in this part and in Parts 211 through 226 of this chapter for Pharma company. (b) The following definitions of …
Read More »Buildings and Facilities in pharma industry as per USFDA
Buildings and Facilities in pharma industry Design and construction features in pharma industry (a) Any building or buildings used in the manufacture, processing, packing, or holding of a drug product shall be of suitable size, construction, and location to facilitate cleaning, maintenance, and proper operations. (b) Any such building shall have adequate space for the …
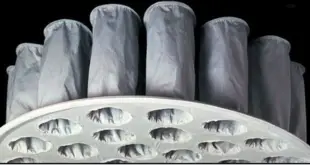
Read More »SOP for cleaning of FBD bag
SOP for cleaning of FBD bag Standard Operating Procedure (SOP) Objective To lay down a procedure for cleaning of FBD bags. Scope This SOP is applicable for the cleaning of FBD bags in the formulation plant. Responsibility Production Operator/ Technician – For cleaning of the equipment. Production Officer/ Executive – …
Read More »SOP for operation of Tablet Inspection Machine
SOP for operation of Tablet Inspection Machine Standard Operating Procedure (SOP) Objective To lay down the procedure for operation of Tablet Inspection Machine. Scope This SOP is applicable for the operation of Tablet Inspection Machine in the formulation plant. Responsibility Production Operator/ Technician – For operation of the machine. Production …
Read More »SOP for operation of Auto coater
SOP for operation of Auto coater Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of Auto coater. Scope This SOP is applicable for operation of Auto coater in the formulation plant. Responsibility Production Operator/ Technician – For operation of the machine. Production Officer/Executive -To maintain proper …
Read More »Document Management System in Quality Assurance Department in Pharma Industry
Document Management System in Quality Assurance Department Standard Operating Procedure (SOP) Objective: To lay down a procedure for Management of Documents in Quality Assurance Department in Pharma Industry Scope: Applicable for Document Management system in Quality Assurance department. Responsibility: Quality Assurance Department Accountability: Head- QA shall be accountable for compliance …
Read More »SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry
SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of rapid mixer granulator with cone mill in Pharma Industry Scope This SOP is applicable for operation of rapid mixer granulator with cone mill …
Read More »Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry
Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for cleaning of Rapid Mixer Granulator with cone mill in Pharma Industry. Scope: This SOP is applicable for the cleaning of Rapid Mixer Granulator with cone mill located in …
Read More »SOP on cleaning of Paste Kettle in Pharma Industry
SOP on cleaning of Paste Kettle in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for cleaning of Paste Kettle in Pharma Industry. Scope This SOP is applicable for the cleaning of Paste Kettle in the formulation plant of Pharma Industry. Responsibility Production Operator/ Technician – …
Read More »SOP for operation of Metal Detector in Pharma Industry
SOP for operation of Metal Detector in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for operation of metal detector in Pharma Industry. Scope: This SOP is applicable for the operation of metal detector to the formulation plant of Pharma Industry. Responsibility: Production Operator/ Technician shall be …
Read More »SOP on Operation of Paste Kettle In Pharma Industry
SOP on the operation of Paste Kettle In the Pharma Industry Objective To lay down the procedure for operation of Paste Kettle In Pharma Industry. Scope This SOP is applicable for operation of Paste Kettle In Pharma Industry. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ …
Read More »Contract production, analysis and other activities as per WHO Guideline in Pharma Industry
Contract production, analysis, and other activities in the Pharma Industry Principle. Contract production, analysis and any other activity covered by GMP must be correctly defined, agreed and controlled in order to avoid misunderstandings that could result in a product, or work or analysis, of unsatisfactory quality. All arrangements for contract …
Read More »Pharma Manufacturing Premises – Risk classification observations
Pharma Manufacturing Premises – Risk classification guide for drug good manufacturing practices observations Risk 1 (critical) observations in Pharma Industry There was no air filtration system to eliminate airborne contaminants likely to be generated during fabrication or packaging. There was generalized malfunctioning of the ventilation system(s), with evidence of widespread …
Read More »Process Validation: General Principles (USFDA) in Pharma Industry
Process Validation: General Principles (USFDA) in Pharma Industry This guidance incorporates principles and approaches that all manufacturers can use to validate manufacturing processes. FDA encourages the use of modern pharmaceutical development concepts, quality risk management, and quality systems at all stages of the manufacturing process lifecycle. The lifecycle concept links …
Read More »Management Review
Management Review Management Review has a systematic approach for quality review as per the Quality Management System (QMS) to monitor product quality, validate the status of products and related processes, identify improvements required in manufacturing processes and systems, and take care of quality risks throughout product life-cycle. Scope of Management …
Read More »SOP on SOP in Industry (Pharmaceuticals Industry)
Standard Operating Procedures- SOP on SOP in Industry (Pharmaceuticals Industry) SOP in Industry stands for “standard operating procedure,” a set of step-by-step instructions for completing a task, An SOP goes beyond being just a procedural document it serves as a reference guide for problem-solving, a tool for ensuring safety, and …
Read More »Line Clearance
line clearance Objective: To lay down the procedure for line clearance of manufacturing and warehouse area. This is to ensure that the start up of any production/ process is free from previous material/ contaminants. Scope: This SOP is applicable for line clearance of production (oral), production (Injection), and warehouse areas …
Read More »PREVENTIVE MAINTENANCE OF PRODUCTION EQUIPMENT
PREVENTIVE MAINTENANCE OF PRODUCTION EQUIPMENT OBJECTIVE; To define the procedure for preventive maintenance of production equipment. SCOPE: This procedure is applicable for preventive maintenance of production equipment. RESPONSIBILITY: This procedure is applicable for preventive maintenance of production equipment. RESPONSIBILITY: Mechanical Engineer ACCOUNTABILITY: Engineer Head ATTACHMENTS: Attachment I – Preventive maintenance …
Read More »