UV efficacy Challenge test Evaluation of ultraviolet irradiation efficacy in an automated system for the aseptic compounding using challenge test Objective Ultraviolet (UV) irradiation efficacy in the intravenous compounding robot APOTECA chemo was evaluated to define the best operative conditions in terms of sterility and time optimization. Design The challenge …
Read More »Sterilization (Introduction, Methods, Definition of Terms)
Sterilization (Introduction, Methods, Definition of Terms) Introduction A major risk of all such medical and surgical instruments procedures is the introduction of pathogens that can lead to infection. Failure to properly disinfect or sterilize equipment carries not only risk associated with breach of host barriers but also risk for person-to-person …
Read More »List of Essential Medicines
List of Essential Medicines (W): Included in WHO Model List of Essential Medicines (I): Included in National List of Essential Medicines (2011), India A Abacavir (ABC) (W) Acenocoumarol (I) Acetazolamide (W,I) Acetic acid (W) Acetylcysteine (W) Acetyl salicylic acid (Aspirin) (W,I) Acriflavin + Glycerine (I) Actinomycin D (Dactinomycin) (W,I) Activated …
Read More »PROTEINS & TYPES OF PROTEINS
PROTEINS Proteins are high molecular weight polyhydroxy peptides containing alpha amino acids joined together by peptide linkage (bond) C―CO―Na. They contain ―C, H, N and S sometimes phosphor also. The molecular weight may range from 6000 to many millions. Proteins are made up of amino acids which from the fundamental …
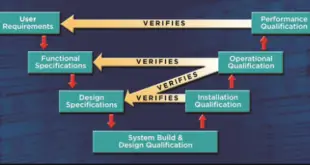
Read More »Qualification & Validation of Equipment, Systems, Utilities in Pharmaceuticals
Qualification & Validation of Equipment Equipment qualification as required by the FDA requires verification documentation to start with the Validation Master Plan (VMP) and flow through a series of documents that define the scope and tasks required to successfully execute your equipment qualification task. The VMP dictates the actions that all persons involved …
Read More »PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM
PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM OBJECTIVE To describe the Performance Qualification procedure to be used during validation of purified water system in order to: a) ensure the system reproducibility over an appropriate time period as per user requirement specifications. b) ensure that the system is showing consistency in producing …
Read More »Cleaning Validation – Glossary of Terms
Cleaning Validation Glossary of Terms 1 Acceptable daily intake An amount of a substance administered or consumed on a daily basis that will not produce a pharmacological or toxic response 2 Analyte Substance for which an analysis is being performed 3 API Active pharmaceutical ingredient 4 Automated cleaning A cleaning …
Read More »Production & purification of drinking-water as per WHO
Production & purification of drinking-water as per WHO General considerations The specifications for WPU found in compendia (e.g. pharmacopoeias) do not define the permissible water purification methods apart from for BWFI. The chosen water purification method or sequence of purification steps must be appropriate to the application in question. The …
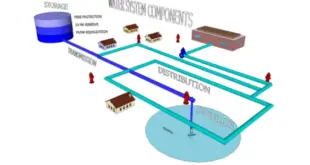
Read More »General principles for pharmaceutical water systems As per WHO
General principles for pharmaceutical water systems Pharmaceutical water production, storage and distribution systems should be designed, installed, commissioned, qualified and maintained to ensure the reliable production of water of an appropriate quality. It is necessary to validate the water production process to ensure the water generated, stored and distributed is …
Read More »Materials in Pharmaceutical as per WHO Guideline
Materials in Pharmaceutical as per WHO Guideline Principle. The main objective of a pharmaceutical plant is to produce finished products for patients’ use from a combination of materials (starting and packaging). Materials include starting materials, packaging materials, gases, solvents, process aids, reagents and labelling materials. No materials used for operations …
Read More »SOP on SOP
SOP on SOP SOP on SOP is a Guidance of SOP that will explain how to draft and prepared the SOP, A standard operating procedure (SOP) is a step-by-step, repeatable process for any routine task. It’s a kind of documentation that prevents stress, mistakes, and miscommunication. SOPs ensure reliability, efficiency, …
Read More »ICH Q8/Q9/Q10 Questions and Answers
ICH Q8/Q9/Q10 1.1 For General Clarification Date of Approval Questions Answers 1.0 June 2009 Is the minimal approach accepted by regulators? Yes. The minimal approach as defined in Q8(R2) (sometimes also called ‘baseline’ or ‘traditional’ approach) is the expectation that is to be achieved for a fully acceptable submission. However, …
Read More »Guidance Document Cleaning Validation
Guidance Document Cleaning Validation Scope Introduction Principles Validation of Cleaning Processes Equipment and Personnel Microbiological Considerations Documentation Analytical Methods Sampling, Rinsing, Rinse Samples and Detergents Establishment of Limits Change Control/revalidation References 3.1 The objective of the cleaning validation is to verify the effectiveness of the cleaning procedure for removal of …
Read More »Technology Transfer in pharmaceutical manufacturing (WHO)
Technology Transfer in pharmaceutical manufacturing (WHO) Introduction Scope Glossary Organization and management Production: transfer (processing, packaging and cleaning) Quality control: analytical method transfer Premises and equipment Documentation Qualification and validation 1.1 Transfer of processes to an alternative site occurs at some stage in the life-cycle of most products, from development, …
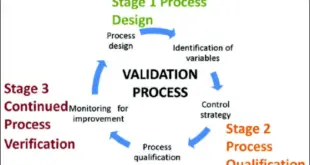
Read More »Process Validation Critical Parameters
Process Validation Critical Parameters Process Validation(FDA Definition) Establishing Documented Evidence, Which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.” Steps in Validating a Process: Develop validation protocol Conduct installation qualification Conduct operational qualification Conduct performance qualification …
Read More »Equipment Status log
Equipment Status log OBJECTIVE: To lay down a procedure of the maintenance of equipment sequential log. SCOPE: This SOP is applicable to all equipment and machines of all manufacturing departments RESPONSIBILITY: Production and Engineering officer/above shall be responsible to follow the procedure mentioned in this SOP. ACCOUNTABILITY : Concerned Department Head and QA Head …
Read More »Cleaning of general item
Cleaning of general item OBJECTIVE: To lay down a procedure to describe the steps to be followed for cleaning general items. SCOPE: Applicable for cleaning all HDPE / SS containers, utensils, scoops, sampling tools and different cleaning aids RESPONSIBILITY: Housekeeping workers, Production Supervisors, Operators, IPQA personnel shall be responsible to follow the …
Read More »NON-CONFORMANCE RESULTS
NON-CONFORMANCE RESULTS OBJECTIVE: To lay down the procedure for define the necessary action against the non-conformance of a product. SCOPE: This procedure is applicable for information, investigation and control for any kind of non- conformance noticed or confirmed in Production, Stores on in others area for its proper investigation RESPONSIBILITY: Officer – …
Read More »Prevention and Product Mix Up
Prevention and Product Mix Up OBJECTIVE: To lay down the Procedure for prevention of Product mix-up. SCOPE: This procedure is applicable for the control and prevention of product mix up during and after the manufacturing of the product . RESPONSIBILITY: QA officer and Production Officer shall be responsible for the …
Read More »GUIDE TO INSPECTIONS VALIDATION OF CLEANING PROCESSES
INTRODUCTION To establish inspection consistency and uniformity by discussing practices that has been found acceptable (or unacceptable). The cleaning validation – to validate the process and collect the scientific data that prove the system consistently does as expected and produce a result that consistently meets predetermined specifications. This guide is …
Read More »