PPT on prevent the spread of COVID 19 infection at the workplace

Investigating Out-of-Specification IDENTIFYING AND ASSESSING OOS TEST RESULTS — PHASE I: LABORATORY INVESTIGATION PHASE II: FULL-SCALE OOS INVESTIGATION

INPROCESS CONTROL OF CAPSULE MANUFACTURING OBJECTIVE: To lay down the procedure for In process Control of Capsule Manufacturing. SCOPE: This SOP covers the responsibility and procedure for In process control during capsule manufacturing. RESPONSIBILITY: In process Quality Assurance Executive/Officer. ACCOUNTABILITY: Quality Assurance Manager. PROCEDURE: Carry out line clearance at each stage of operation prior to…

OBJECTIVE:To describe the calibration procedure for distribution of temperature within the chamber of Refrigerator. SCOPE: his SOP shall be applicable for Calibration of Refrigerator at Pharmaceutical Industries. RESPONSIBILITY : Quality Control Executive/Officer. ACCOUNTABILITY Head Quality Control PROCEDURE : Operate the refrigerator as per Standard Procedure. Insert the probe of…
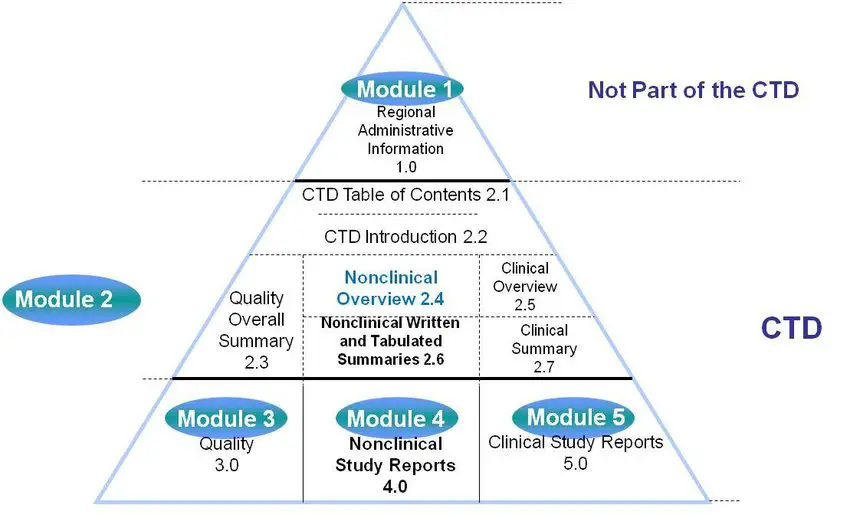
ANDA Submissions — Content and CTD Format (USFDA) TABLE OF CONTENTS CTD FORMAT A. Module 1 – Administrative Information Forms and Cover Letter Administrative Information References Other Correspondence Labeling B.Module 2 – CTD Summaries Quality Overall Summary Clinical Summary C.Module 3 – Quality Drug Substance Drug Product Appendices Regional Information Literature References D.Module 4 –…
Read More “ANDA Submissions — Content and CTD Format (USFDA)” »
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Operation, Calibration, and Cleaning of the Conductivity meter .2.0 SCOPE:This procedure shall apply to quality control of Pharmaceutical Company for Operation, Calibration, and Cleaning of the Conductivity meter .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads…
Read More “SOP for Operation, Calibration, and Cleaning of the Conductivity meter” »
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Checklist for microbiology data Review .2.0 SCOPE:This procedure shall apply to quality Control(Microbiology lab) of Pharmaceutical Company for microbiology data Review .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of the…
Read More “SOP for Checklist for microbiology data Review.” »
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Checklist for Analytical Raw Data Review (Chemical). 2.0 SCOPE:This procedure shall apply to Quality Ccontrol of Pharmaceutical Company for Analytical Raw Data Review (Chemical) .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for…
Read More “SOP for Checklist for Analytical Raw Data Review (Chemical)” »
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Calibration of UV spectrophotometer.2.0 SCOPE:This procedure shall apply for Calibration of UV spectrophotometer in quality control departments .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of the procedure. 4.0 ACCOUNTABILITY: QC…
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. 2.0 SCOPE:This procedure shall apply for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for…
Read More “SOP for Operation and Calibration of Total Organic Carbon (TOC)” »
Objective: To lay down a procedure for Calibration of Gas Chromatography (GC). Scope: This Standard Operating Procedure is applicable for for Calibration of Gas Chromatography (GC). 3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of the procedure. 4.0 ACCOUNTABILITY: Production and QA…
Read More “SOP for for Calibration of Gas Chromatography (GC)” »
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Acceptable Quality Level sampling for Tablets and capsule 2.0 SCOPE:This procedure shall apply to formulation plant of Pharmaceutical Company that manufacture, package, test, and store or distribute drug products. 3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department…
Read More “SOP for Acceptable Quality Level sampling for Tablets and capsule” »
STABILITY STUDY MANAGEMENT Stability Study Management – To lay down the procedure to conduct stability studies of the drug product (dosage forms)/ Drug substances (API). This Procedure describes the procedure for assigning the stability of drug substances (Active pharma ingredients) and drug products (Packed finished dosage forms). The purpose of stability studies is to obtain…

Objective To lay down the procedure for in-process control of oral drug products during manufacturing & Packing. Scope This procedure is applicable for in-process sampling, analysis, and reporting to be carried out during manufacturing and packing of drug products at the formulation Plant. Responsibility Quality Assurance and production personnel shall responsible to follow the procedure…
Read More “In-process control of oral drug product during manufacturing & Packing” »

OBJECTIVE To lay down a procedure for sampling of packaging materials. SCOPE To describe the procedure for sampling of Primary packing materials i.e. Aluminium Foil, Blister Aluminium Foil, PVC Film etc. and secondary packing materials i.e. Cartons, labels, Leaflet shipper etc. RESPONSIBILITY Quality Control Executive/Officer ACCOUNTABILITY Quality Assurance Manager PROCEDURE On receipt of the Goods…

1.0 OBJECTIVE To lay down the procedure for operation and cleaning of unit dose sampler. 2.0 SCOPE This SOP shall be applicable for IPQA area in Quality Assurance. 3.0 RESPONSIBILITY In process Quality Assurance Executive /Officer 4.0 ACCOUNTABILITY Head Quality Assurance 5.0 PROCEDURE FOR OPERATING 5.1 Check the Status label of the sampler. 5.2 …
Read More “PROCEDURE FOR OPERATION OF UNIT DOSAGE SAMPLER” »