Pharmaceutical Quality Assurance -Drug and Therapeutics Committee Training Course CONTENTS Pharmaceutical Quality Assurance Acknowledgment Purpose and Content Objectives Outline Key Definitions Introduction Determinants or Aspects of Medicine Quality Critical Elements of a Comprehensive Quality Assurance Program How Is Quality Assessed? How Is Quality Assured? Who Ensures Medicine Quality? Pharmaceutical Quality …
Read More »MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE AS PER INTERNATIONAL PHARMACOPOEIA
MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE AS PER INTERNATIONAL PHARMACOPOEIA Definition Liquid preparations for oral use are usually solutions, emulsions or suspensions containing one or more active ingredients in a suitable vehicle, they may in some cases consist simply of a liquid active ingredient used as such. Liquid preparations …
Read More »SOP on Testing Efficacy of Disinfectants
SOP on Testing Efficacy of Disinfectants. Objective: To lay down a procedure for testing efficacy of Disinfectants. Scope : This SOP is applicable for testing of efficacy of disinfectant solutions used in microbiology laboratory and production area. Responsibility Microbiologist Head-Microbiology Accountability Head-Quality Control Head-Quality Assurance Procedure Prepare Culture suspension of …
Read More »Lorazepam Aristo 2.5 mg tablets
Lorazepam Aristo 2.5 mg tablets Active ingredient – lorazepam Legal Category : POM: Prescription only medicine 1. Name of the medicinal product Lorazepam Aristo 2.5 mg tablets 2. Qualitative and quantitative composition One tablet contains 2.5 mg lorazepam. Excipients with known effect Each tablet contains 116.7 mg lactose (as lactose …
Read More »Aciclovir 400 mg Tablets
Aciclovir 400 mg Tablets Active ingredient – Aciclovir Legal Category -POM: Prescription only medicine 1. Name of the medicinal product Aciclovir 400 mg Tablets 2. Qualitative and quantitative composition Each 400 mg tablet contains 400 mg aciclovir. For the full list of excipients, see section 6.1 3. Pharmaceutical form Tablet …
Read More »Furosemide 40 mg Tablets
Furosemide 40 mg Tablets Active ingredient – Furosemide Legal Category – POM: Prescription only medicine 1. Name of the medicinal product Furosemide 40mg Tablets 2. Qualitative and quantitative composition Each tablet contains Furosemide 40mg. For a full list of excipients, see section 6.1. 3. Pharmaceutical form Tablet White to off white, …
Read More »SOP on Personnel Qualification for Aseptic Processing and Sterility Testing Area
Personnel Qualification for Aseptic Processing and Sterility Testing Area Objective: To lay down the procedure for Personnel Qualification to enter and work in the Aseptic Processing area and Sterility Testing Area. Scope: This SOP is applicable for Personnel Qualification to work in Aseptic Processing and Sterility testing Area Procedure: For …
Read More »SOP on Personnel Qualification for Aseptic Processing and Sterility Testing Area
Objective: To lay down the procedure for Personnel Qualification to enter and work in Aseptic Processing area and Sterility Testing Area. Scope: This SOP is applicable for Personnel Qualification to work in Aseptic Processing and Sterility testing Area. Responsibility: Chemist or above of Microbiology Laboratory. Head – Microbiology Section. Accountability: …
Read More »SOP on Viable Particle Monitoring of Drain Points– Sterile Product Manufacturing Facility
Objective: To lay down the procedure for Viable Particulate Monitoring of Drain Points – Sterile Product Manufacturing Facility Scope: This SOP is applicable for Viable Particle Monitoring of Drain Points – Sterile Product Manufacturing Facility Responsibility: Officer or above of Microbiology Laboratory. Head – Microbiology section / Nominee. Accountability: Head …
Read More »SOP on calibration of pH meter
Objective: To lay down a procedure for calibration of pH Meter. Scope: This SOP is applicable for calibration of pH Meter in quality control laboratory. Responsibility: Officer or above of QC laboratory is responsible for calibration of pH Meter Head Quality / nominee of QC department is responsible for reviewing …
Read More »Aceclofenac 100 mg Film-coated Tablets
Aceclofenac 100 mg Film-coated Tablets 1. Name of the medicinal product Aceclofenac 100 mg Film-coated Tablets 2. Qualitative and quantitative composition Each film-coated tablet contains 100 mg of aceclofenac For the full list of excipients, see section 6.1. 3. Pharmaceutical form Film-coated tablet. White, round, biconvex film-coated tablets, 8 mm …
Read More »Q7 GMP Guidance for Active Pharmaceutical Ingredients
Guidance for Industry II. QUESTIONS AND ANSWERS A. Scope (1) 1.1: Should GMP according to ICH Q7 be applied for manufacturing steps before the defined API starting material, i.e., steps not identified in grey in Table 1? ICH Q7 does not apply to steps prior to the introduction of the …
Read More »Actelsar HCT tablets
Actelsar HCT tablets 1. Name of the medicinal product Actelsar HCT 40 mg/12.5 mg tablets Actelsar HCT 80 mg/12.5 mg tablets 2. Qualitative and quantitative composition Actelsar HCT 40 mg/12.5 mg tablets Each tablet contains 40 mg telmisartan and 12.5 mg hydrochlorothiazide. Actelsar HCT 80 mg/12.5 mg tablets Each tablet …
Read More »Actos tablets
Actos tablets 1. Name of the medicinal product Actos 15 mg tablets Actos 30 mg tablets Actos 45 mg tablets 2. Qualitative and quantitative composition Actos 15 mg tablets Each tablet contains 15 mg of pioglitazone (as hydrochloride). Excipient with known effect Each tablet contains 92.87 mg of lactose monohydrate …
Read More »ABILIFY 1 mg/mL oral solution
ABILIFY 1 mg/mL oral solution 1. Name of the medicinal product ABILIFY 1 mg/mL oral solution 2. Qualitative and quantitative composition Each mL oral solution contains 1 mg of aripiprazole. Excipients with known effect (per mL) 200 mg fructose, 400 mg sucrose, 1.8 mg methyl parahydroxybenzoate (E218), 0.2 mg propyl …
Read More »Sampling and testing of Sterile surgical Gloves
Objective: To lay down the procedure for Sampling and testing of sterile surgical gloves. Scope: This SOP is applicable for sampling and testing of sterile surgical gloves. Responsibility: Personnel (Microbiology-QC) shall be responsible for sampling and testing of sterile surgical gloves. Executive/ Officer shall be responsible for ensuring the compliance …
Read More »STABILITY TESTING OF NEW DRUG PRODUCTS Q1A(R2)
STABILITY TESTING OF NEW DRUG PRODUCTS Q1A(R2) Drug Product General Photostability Testing Selection of Batches Container Closure System Specification Testing Frequency Storage Conditions Stability Commitment Evaluation Statements/Labeling Drug Product General The design of the formal stability studies for the drug product should be based on knowledge of the behavior and …
Read More »STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2)
STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2) COVER NOTE FOR REVISION OF Q1A(R) The changes made in Q1A(R) that result from adoption of ICH Q1F “Stability Data Package for Registration Applications in Climatic Zones III and IV”. These changes are: The intermediate storage condition has been changed from 30°C ± …
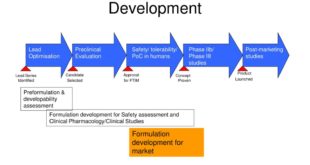
Read More »Pharmaceutical Development as per USFDA
PHARMACEUTICAL DEVELOPMENT I. INTRODUCTION II. PHARMACEUTICAL DEVELOPMENT A. Components of the Drug Product 1. Drug Substance 2. Excipients B. Drug Product 1. Formulation Development 2. Overages 3. Physicochemical and Biological Properties C. Manufacturing Process Development D. Container Closure System E. Microbiological Attributes F. Compatibility III. GLOSSARY INTRODUCTION The Pharmaceutical …
Read More »