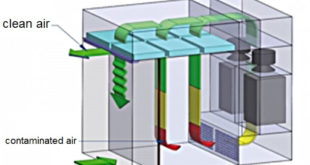
1.0 OBJECTIVE To lay down the procedure for operation and cleaning of unit dose sampler. 2.0 SCOPE This SOP shall be applicable for IPQA area in Quality Assurance. 3.0 RESPONSIBILITY In process Quality Assurance Executive /Officer 4.0 ACCOUNTABILITY Head Quality Assurance 5.0 PROCEDURE FOR OPERATING 5.1 Check the Status …
Read More »Batch Release Statement for Pharmaceutical Product
Performace Qualification Protocol of Dispensing Booth
Performance Qualification Protocol of Dispensing Booth
Read More »Procedure for Microbiological Monitoring of Purified water
SOP In-process Control of Packing Lines
SOP on Sampling of Intermediates and Finished Products
SOP On In-process Control During Tablets Manufacturing
SOP to prevent the spread of COVID 19 infection at workplace
SOP to prevent the spread of COVID 19 infection at workplace
Read More »SOP on Line Clearance
SOP on QUALITY RISK MANAGEMENT (RISK ASSESSMENT)
1.0 Objective: To provide a procedure for carrying out Risk assessment, evaluation, mitigation and review of risk by employing appropriate tool of Quality Risk Management Process. 2.0 Scope: Applicable to different aspects of pharmaceutical quality like development, manufacturing, testing, distribution, inspection and submission/review processes throughout the life cycle of drug …
Read More »SOP On Risk Assessment Sample Format on Tablets Manufacturing Process
FDA – Warning Letter
FDA – Warning Letter September 10, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more FDA warning Letter …
Read More »Pharma FDA Warning Letter for Derma Pharm A/S MARCS-CMS – November 26
Teligent Pharma, Inc. MARCS-CMS 587592 — November 26, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more …
Read More »Pharma FDA Warning Letter for OHM Pharma, Inc -November 19
Pharma FDA Warning Letter for OHM Pharma, Inc MARCS-CMS 586428 — November 19, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed …
Read More »Pharma FDA Warning Letter for Alkermes, Inc.
For FDA Warning Letter Click Here – Alkermes, Inc. MARCS-CMS 597260 — December 02, 2019 Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more USFDA …
Read More »FDA -WARNING LETTER -March 30, 2023
FDA -WARNING LETTER -March 30, 2023 The U.S. Food and Drug Administration (FDA) inspected your drug manufacturing facility, ALI Pharmaceutical Manufacturing, LLC, FEI 1920841, at 4410 S. 102nd Street, Omaha, from September 26 to October 3, 2022. This warning letter summarizes significant deviations from current good manufacturing practice (CGMP) for …
Read More »Pharma FDA Warning Letter October 4, 2023
Pharma FDA Warning Letter October 4, 2023 The U.S. Food and Drug Administration (FDA) inspected your drug manufacturing facility, Handock Cosmetics Co., Ltd., FEI 3007295883, at 19 Eunbong-ro, Namdong-gu, Incheon 21634, from March 20 to March 24, 2023. This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) …
Read More »Pharma FDA Warning Letter -October 16, 2023
Pharma FDA Warning Letter -October 16, 2023 The U.S. Food and Drug Administration (FDA) inspected your drug manufacturing facility from April 26 to May 9, 2022. This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations for finished pharmaceuticals. See Title 21 Code of Federal Regulations (CFR), …
Read More »Pharma FDA Warning Letter -October 2, 2023
Pharma FDA Warning Letter -October 2, 2023 The U.S. Food and Drug Administration (FDA) inspected your drug manufacturing facility, Seoul Cosmetics Co., Ltd., FEI 3007253462, at 12, Namdongdong-ro 63beon-Gil, Namdong-gu, from January 30 to February 3, 2023. This warning letter summarizes significant violations of Current Good Manufacturing Practice (CGMP) regulations …
Read More »FDA Warning Letter -October 2, 2023
FDA Warning Letter -October 2, 2023 This is to advise you that the United States (U.S.) Food and Drug Administration (FDA) recently reviewed your website at the Internet address www.gorillahealing.com and has observed that your website introduces into interstate commerce misbranded and unapproved new drugs in violation of sections 301(a), …
Read More »