Read More »
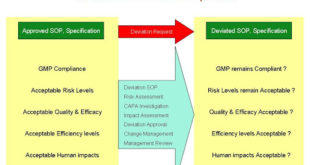
Cleaning Validation as per APIC Guideline
PPT on prevent the spread of COVID 19 infection at the workplace
Investigating Out-of-Specification
Investigating Out-of-Specification IDENTIFYING AND ASSESSING OOS TEST RESULTS — PHASE I: LABORATORY INVESTIGATION PHASE II: FULL-SCALE OOS INVESTIGATION
Read More »INPROCESS CONTROL OF CAPSULE MANUFACTURING
INPROCESS CONTROL OF CAPSULE MANUFACTURING OBJECTIVE: To lay down the procedure for In process Control of Capsule Manufacturing. SCOPE: This SOP covers the responsibility and procedure for In process control during capsule manufacturing. RESPONSIBILITY: In process Quality Assurance Executive/Officer. ACCOUNTABILITY: Quality Assurance Manager. PROCEDURE: Carry out line clearance at each …
Read More »PROCEDURE FOR CALIBRATION OF REFRIGERATOR
OBJECTIVE:To describe the calibration procedure for distribution of temperature within the chamber of Refrigerator. SCOPE: his SOP shall be applicable for Calibration of Refrigerator at Pharmaceutical Industries. RESPONSIBILITY : Quality Control Executive/Officer. ACCOUNTABILITY Head Quality Control PROCEDURE : Operate the refrigerator as per Standard …
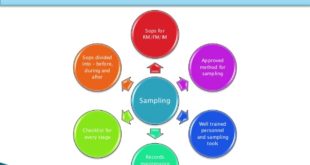
Read More »USFDA GUIDELINE FOR DRUG QUALITY SAMPLING AND TESTING (DQST)- HUMAN DRUGS
USFDA GUIDELINE FOR DRUG QUALITY SAMPLING AND TESTING (DQST)- HUMAN DRUGS
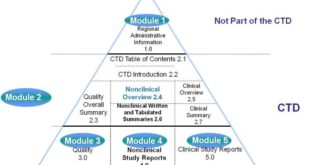
Read More »ANDA Submissions — Content and CTD Format (USFDA)
ANDA Submissions — Content and CTD Format (USFDA) TABLE OF CONTENTS CTD FORMAT A. Module 1 – Administrative Information Forms and Cover Letter Administrative Information References Other Correspondence Labeling B.Module 2 – CTD Summaries Quality Overall Summary Clinical Summary C.Module 3 – Quality Drug Substance Drug Product Appendices Regional Information …
Read More »Checklist in Quality control for Audit Preparation of FDA & USFDA
Checklist in Quality control for Audit Preparation of FDA & USFDA Personnel Organogram of Quality control departments List of Employee and education details Individual Job Description Training Record of Individual employee as per individual Job Responsibility and Training Log and training assessments records Analyst Qualification records as per individual Job Responsibility and …
Read More »LINE CLEARANCE CHECKLIST FOR VACUUM CLEANER
LINE CLEARANCE CHECKLIST FOR VACUUM CLEANER Dosage Form: Date / Time: Product: Batch No. : Previous Product: Batch No. : Vacuum cleaner ID No.: Stage areas / room & equipment Checks Checked by Production Date & Time Counter checked By Quality Assurance Date & Time Vacuum cleaner Equipment ID No.: …
Read More »LINE CLEARANCE CHECKLIST OF AREA & EQUIPMENT FOR PACKING LINE
LINE CLEARANCE CHECKLIST OF AREA & EQUIPMENT FOR PACKING LINE Dosage Form: Date / Time: Product: Batch No. : Previous product: Batch No. : Stage areas / Equipment Name Checks Checked by Production Date & Time Counter checked By Quality Assurance Date & Time PACKING [Released / …
Read More »BATCH MANUFACTURING RECORD (BMR)
BATCH MANUFACTURING RECORD (BMR) TABLE OF CONTENT GENERAL PROCESSING INSTRUCTION LIST OF EQUIPMENT USED DISPENSING OF RAW MATERIAL GRANULATION COMPRESSION COATING TABLET INSPECTION DOCUMENT RECONCILIATION BMR REVIEW BATCH RELEASE 1.0 GENERAL PROCESSING INSTRUCTION All the Activities shall be performed as per current SOPs. Follow GMP compliance throughout the manufacturing process. …
Read More »BATCH PACKING RECORD (BPR)
BATCH PACKING RECORD (BPR) BATCH PACKING RECORD (BPR) BMR Title : BPR for Tablet Document No. : Batch Size : Page No. : 1 of 15 Effective Date : Review Date : Product Name : Generic Name : Labels Claim) : Each _____________________________________ …
Read More »USFDA – INTRODUCTION TO DRUG REGULATION
USFDA – INTRODUCTION TO DRUG REGULATION Basic Mission Safety Efficacy Drugs Medical Devices Truthful Labeling Adulteration Misbranding FDA’s Structure and Organization The Commissioner President Appoints, Senate Confirms 4th Tier in HHS Not an Independent Agency Insulation From Politics (Old Days) Few Political Appointees Scientific Basis of Its Decisions Visibility Protects …
Read More »USFDA Foreign Priorities, Inspections and Compliance
USFDA Foreign Priorities, Inspections and Compliance Priorities Challenges of globalization cGMP deficiencies Comparison Post inspection regulatory actionsAdvance Regulatory Science: the science of developing new tools, standards and approaches to assess the safety and effectiveness, quality and performance of FDA-regulated productsStrengthen the safety and integrity of the global supply chain A …
Read More »USFDA Regulatory Inspection Do’s and Dont’s
USFDA Regulatory Inspection Do’s and Dont’s FDA Audit – The Do and Don’t List Pre-inspection Do’s: • Have a Company Inspection Manual • Have a trained Company Inspection Team • Identify what FDA (or the state) may inspect • Be familiar with relevant sections of FDA’s Investigations Operations Manual. Company …
Read More »DATA INTEGRITY: ALCOA AND ALCOA PLUS
DATA INTEGRITY: ALCOA AND ALCOA PLUS The guidance has been written to apply to on-site inspections of those sites performing manufacturing (GMP) and distribution (GDP) activities. The principles within this guide are applicable for all stages throughout the product lifecycle. The guide should be considered as a non-exhaustive list of areas to be …
Read More »Data Integrity: TGA Expectations
Data Integrity: TGA Expectations Discussion Topics What is Data Integrity? • Global/Australian/US FDA Environments • Data Integrity General Examples • Basic Data Integrity Expectations • ALCOA Principles • TGA Licensed Manufacturers Expectations • Conclusions What is data integrity? • The extent to which all data are complete, consistent and accurate throughout …
Read More »Quality Metrics
Quality Metrics Objectives • Pharmaceutical Quality for the 21st Century • Why Quality Metrics (QM)? • Initial draft of the QM Guidance • Key Features of the QM Revised Draft Guidance • Phased-In Approach and Benefits to Participants • How FDA Intends to Use Metrics Data Pharmaceutical Quality for 21st Century …
Read More »Types of Glass used in Pharmaceutical Industries
Types of Glass used in Pharmaceutical Industries Parenteral Use Type I Glass: Highly Resistant Borosilicate. Used for Buffered and Unbuffered aqueous solution.Type II Glas: Highly Resistant Sodalime glass. The buffered aqueous solution below pH 7.0Type III Glass: Moderately Resistant Sodalime glass. Used for dry powder and oily solution.Non-Parenteral Use Type …
Read More »