URS of De-Dusting Machine S. No. Table of Contents 1.0 General 2.0 Salient Features 3.0 Operational Requirements 4.0 Utilities 5.0 Maintenance 6.0 Commissioning and Documentation 7.0 Training 8.0 Packaging 9.0 Deviations 10.0 Delivery TECHNICAL S. No. Parameters Required Specifications 1. 1.1 General Equipment No. 1.2 Description Portable unit for Tablets …
Read More »SOP on house keeping, cleaning and sanitization of general area in Pharmaceutical company
SOP on housekeeping, cleaning, and sanitization of the general area in a Pharmaceutical company. Objective: To lay down a procedure for Good House Keeping, cleaning, and sanitization of the General Area. Scope: This procedure is applicable for cleaning and sanitization of General areas other than the manufacturing area of the …
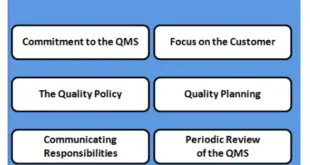
Read More »QUALITY SYSTEMS MODEL (Resources)IN PHARMACEUTICAL INDUSTRY (Part – II)
QUALITY SYSTEMS MODEL The goal is to describe a model for use in pharmaceutical manufacturing that can help manufacturers comply with the CGMP regulations. It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources. However, we believe …
Read More »Pharma WhatsApp Groups for Latest Pharma updates
Dear All Please find the Pharma WhatsApp Groups for Latest Pharma Jobs, Pharma news, and Pharma Posts. Please Click the below link and Join the group easily Pharma Post and Jobs 01 https://chat.whatsapp.com/Dy1GkgHZloYJPsfzIC6tnZ Pharma posts and jobs 25 https://chat.whatsapp.com/JZD6QCCtaGpGdWYu3oU3iy
Read More »QUALITY SYSTEMS MODEL IN PHARMACEUTICAL INDUSTRY (Part – I)
QUALITY SYSTEMS MODEL The goal is to describe a model for use in pharmaceutical manufacturing that can help manufacturers comply with the CGMP regulations. It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources. However, we believe …
Read More »CGMPS AND THE CONCEPTS OF MODERN QUALITY SYSTEMS
CGMPS AND THE CONCEPTS OF MODERN QUALITY SYSTEMS Quality should be built into the product, and testing alone cannot be relied on to ensure product quality. Several key concepts are critical for any discussion of modern quality systems. The following concepts are used throughout this guidance as they relate to …
Read More »What are good documentation practices & how can they best be implemented?
What are good documentation practices & how can they best be implemented? Good documentation practice (commonly abbreviated GDP, recommended to abbreviate as GDocP to distinguish from “good distribution practice” also abbreviated GDP) is a term in the pharmaceutical and medical device industries to describe standards by which documents are created and maintained. While some GDP / GDocP standards …
Read More »Drug Master Files (DMFs) and it submission
Drug Master Files (DMFs) and it submission Drug master files (DMFs) are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. They: Allow parties to reference material without disclosing DMF contents to those …
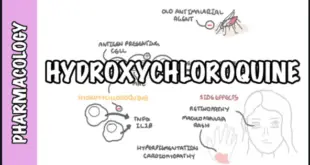
Read More »Hydroxychloroquine and Antimalarial Drugs (united healthcare)
Hydroxychloroquine and Antimalarial Drugs (united healthcare) These are antimalarial drugs found to induce remission in upto 50% patients of Rheumatoid Arthritis (RA), but take 3–6 months. Their advantage is relatively low toxicity, but efficacy is also low; bony erosions are not prevented. Their mechanism of action is not known, however, …
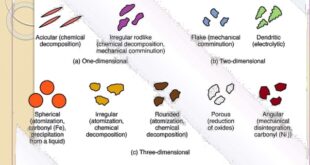
Read More »Characterization of Fine Particle in Formulation of drugs
Characterization of Fine Particle in Formulation of drugs Parameters those are measured: (i) Particle size and size-distribution (ii) Shape of the particle (iii) Surface morphology of the particles (iii) Zeta potential Instrumental Methods of Particle Size Characterization: (i) Light Microscope: • First a standard graticule (BS 3625) is standardized with …
Read More »PROCESSING OF PARENTERAL PREPARATION
PROCESSING OF PARENTERAL PREPARATION The following steps are involved in the processing of parenteral preparation: 1. Cleaning of containers, closures, and equipment. 2. Collection of materials. 3. Preparation of parenteral products. 4. Filtration. 5. Fill the preparation in the final container. 6. Sealing the container. 7. Sterilization 8. Evaluation of …
Read More »Formulation of Suspensions
Formulation of Suspensions Biphasic liquids such as suspensions and emulsions are unique dosage forms because many of their properties are due to the presence of a boundary region between two phases. In suspensions, a liquid and an insoluble solid meet to form an interface. In the case of emulsions, two …
Read More »Determination of equilibrium solubility of a drug
Determination of equilibrium solubility of a drug Determination of equilibrium solubility of a drug: The drug is dispersed in a solvent. The suspension is agitated at a steady temperature. Samples of the suspension are withdrawn as a function of time, clarified by centrifugation,and assayed to establish a plateau concentration. Solvents …
Read More »QC SOPs List
QC SOPs List SNo. SOPs Title 1.0 SOP on entry & exit procedure in Quality Control Department 2.0 SOP on sampling of raw material. 3.0 SOP on intermediate and finished product analysis and approval 4.0 SOP on analysis of sample by contract laboratory 5.0 SOP on sampling procedure of packaging …
Read More »Quality Assurances SOPs List
Quality Assurances SOPs List SNo. SOP Title 01 SOP on validation master plan 02 SOP on Function of quality assurance department 03 SOP on periodic physical observation of control sample 04 SOP on job responsibility of personnel working in quality assurance. 05 SOP on in-process sampling and analysis of oral …
Read More »PROBLEMS AND REMEDIES FOR TABLET COATING
PROBLEMS AND REMEDIES FOR TABLET COATING Blistering It is local detachment of film from the substrate forming blister. Reason: Entrapment of gases in or underneath the film due to overheating either during spraying or at the end of the coating run. Cause and Remedy of Blistering: Cause: Effect of temperature …
Read More »VALIDATION AND QUALIFICATION OF WATER PURIFICATION,STORAGE,AND DISTRIBUTION SYSTEMS AS PER USP
Purified Water Establishing the Quality of pharmaceutical water purification,storage,and distribution systems requires an appropriate period of monitoring and observation. However, it is more difficult to meet established microbiological quality criteria consistently. A typical monitoring and observation program involves intensive daily sampling and testing of major process points for at least …
Read More »Lubricants and Glidants used in Tablet Formulations
Tablets Lubricants Lubricants and Glidants used in Tablet Formulations are: • Magnesium stearate • Stearic acid • Sodium stearyl fumarate • Hydrogenated vegetable oil • PEG 4000, 6000 • Hexagonal boron nitride • DL-Leucine • Sodium lauryl sulfate • Gliceryl behenate • Sodium benzoate • Colloidal silicone dioxide • Talc …
Read More »Preformulation stability studies
Stability Analysis Preformulation stability studies are the first quantitative assessment of chemical stability of a new drug. This may involve 1. Stability study in toxicology formulation 2. Stability study in solution state 3. Stability study in solid state. Stability Study in Toxicology Formulation: A new drug is administered to animals …
Read More »Powder Particle Size Determination Methods for tablets
Particle Size Determination Methods: 1. Sieving 2. Microscopy 3. Sedimentation rate method 4. Light energy diffraction 5. Laser holography 6. Cascade impaction 1. Sieving method: • Range: 50 – 150 μm • Simple, inexpensive • If powder is not dry, the apertures get clogged. 2. Microscopy: • Range: 0.2 – …
Read More »