1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Calibration of UV spectrophotometer.2.0 SCOPE:This procedure shall apply for Calibration of UV spectrophotometer in quality control departments .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of …
Read More »SOP for Operation and Calibration of Total Organic Carbon (TOC)
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. 2.0 SCOPE:This procedure shall apply for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department …
Read More »SOP for for Calibration of Gas Chromatography (GC)
Objective: To lay down a procedure for Calibration of Gas Chromatography (GC). Scope: This Standard Operating Procedure is applicable for for Calibration of Gas Chromatography (GC). 3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of the procedure. …
Read More »SOP for Acceptable Quality Level sampling for Tablets and capsule
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Acceptable Quality Level sampling for Tablets and capsule 2.0 SCOPE:This procedure shall apply to formulation plant of Pharmaceutical Company that manufacture, package, test, and store or distribute drug products. 3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention …
Read More »STABILITY STUDY MANAGEMENT
STABILITY STUDY MANAGEMENT Stability Study Management – To lay down the procedure to conduct stability studies of the drug product (dosage forms)/ Drug substances (API). This Procedure describes the procedure for assigning the stability of drug substances (Active pharma ingredients) and drug products (Packed finished dosage forms). The purpose of …
Read More »In-process control of oral drug product during manufacturing & Packing
Objective To lay down the procedure for in-process control of oral drug products during manufacturing & Packing. Scope This procedure is applicable for in-process sampling, analysis, and reporting to be carried out during manufacturing and packing of drug products at the formulation Plant. Responsibility Quality Assurance and production personnel shall …
Read More »SAMPLING OF PACKING MATERIALS
OBJECTIVE To lay down a procedure for sampling of packaging materials. SCOPE To describe the procedure for sampling of Primary packing materials i.e. Aluminium Foil, Blister Aluminium Foil, PVC Film etc. and secondary packing materials i.e. Cartons, labels, Leaflet shipper etc. RESPONSIBILITY Quality Control Executive/Officer ACCOUNTABILITY Quality Assurance Manager PROCEDURE …
Read More »PROCEDURE FOR OPERATION OF UNIT DOSAGE SAMPLER
1.0 OBJECTIVE To lay down the procedure for operation and cleaning of unit dose sampler. 2.0 SCOPE This SOP shall be applicable for IPQA area in Quality Assurance. 3.0 RESPONSIBILITY In process Quality Assurance Executive /Officer 4.0 ACCOUNTABILITY Head Quality Assurance 5.0 PROCEDURE FOR OPERATING 5.1 Check the Status …
Read More »Batch Release Statement for Pharmaceutical Product
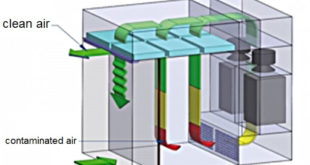
Performace Qualification Protocol of Dispensing Booth
Dispensing Booth A dispensing booth is a kind of laminar flow filtration cabin that is employed in dispensing, sampling, and weighing powders and chemicals in the pharma, biotech, and chemical industries. Such units work on reverse laminar flow working principle and protect operators, products/samples, and the surrounding environment. The dispensing …
Read More »Procedure for Microbiological Monitoring of Purified water
SOP In-process Control of Packing Lines
SOP on Sampling of Intermediates and Finished Products
SOP On In-process Control During Tablets Manufacturing
SOP to prevent the spread of COVID 19 infection at workplace
SOP to prevent the spread of COVID 19 infection at workplace
Read More »SOP on Line Clearance
SOP on QUALITY RISK MANAGEMENT (RISK ASSESSMENT)
1.0 Objective: To provide a procedure for carrying out Risk assessment, evaluation, mitigation and review of risk by employing appropriate tool of Quality Risk Management Process. 2.0 Scope: Applicable to different aspects of pharmaceutical quality like development, manufacturing, testing, distribution, inspection and submission/review processes throughout the life cycle of drug …
Read More »SOP On Risk Assessment Sample Format on Tablets Manufacturing Process
FDA – Warning Letter
FDA – Warning Letter September 10, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more FDA warning Letter …
Read More »Pharma FDA Warning Letter for Derma Pharm A/S MARCS-CMS – November 26
Teligent Pharma, Inc. MARCS-CMS 587592 — November 26, 2019 For FDA Warning Letter Click Here – Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the letter. For more …
Read More »